
Environmental factors significantly influence the rising rates of type 2 diabetes in children.

Environmental factors significantly influence the rising rates of type 2 diabetes in children.
The agency announced that human clinical trials will no longer be required in lieu of a comparative analytical assessment to determine biosimilar equivalency with its reference product.

Liposomal vitamin C enhances bioavailability, offering superior absorption and antioxidant benefits compared with traditional forms, promising better health outcomes.

Heavy workloads, lack of recognition, and stressful environments fuel emotional exhaustion, turnover, and patient safety concerns.

Missteps in prescribing and counseling can escalate into catastrophic harm and significant financial liability.

Recent research reveals rising cancer rates among younger and older adults, linking obesity to increased incidence.

Pharmacist Care Day showcased UPMC Health Plan’s commitment to whole-person care through free health services, pharmacist-led outreach, and strong community collaborations in Pittsburgh.

Christina Barrington, PharmD, champions pharmacists' vital role in enhancing health care access and quality, advocating for their recognition as essential providers.

In Danish athletes, long-term anabolic androgenic steroid use was associated with progressively worsened cardiovascular outcomes.

The FDA approves a simplified single-injection treatment for ulcerative colitis, enhancing patient experience with monthly dosing convenience.

A new low-carbon albuterol inhaler shows therapeutic equivalence to original options, suggesting reduced emissions and improved sustainability for patients with asthma and other respiratory conditions.

Explore the role of pharmacists in managing menopause-related vasomotor symptoms with innovative non-hormonal therapies like Elinzanetant.
Total cholesterol and triglyceride levels were linked to an increased risk of Alzheimer-related declines in cognitive function, whereas high-density lipoprotein cholesterol provided a protective effect.
Lawmakers weigh cost concerns, clinical outcomes, and regulatory barriers as access to these medications evolves.

Explore the impact of extrapyramidal symptoms on patients, including drug-induced parkinsonism and dystonia, and the importance of early detection and management.

Explore effective non-hormonal treatment options for menopause-related vasomotor symptoms, including hot flashes and night sweats, with insights from pharmacists.

The FDA updates sotatercept's label, enhancing treatment options for pulmonary arterial hypertension and reducing severe clinical events.
Research highlights the link between earlier menopause, cardiac function, and brain health, emphasizing the need for sex-specific dementia risk strategies.

Research highlights the impact of provider type on menopause treatment, revealing a need for standardized education to improve care quality for women.
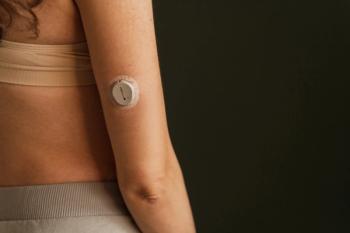
Language preference significantly impacts access to continuous glucose monitors for type 2 diabetes, revealing critical disparities in diabetes care.

Elinzanetant, the first FDA-approved nonhormonal treatment for menopause-related vasomotor symptoms, offers women a new, effective relief option.

Explore the promising data on elinzanetant for managing menopause-related vasomotor symptoms, highlighting its tolerability and minimal side effects.

A heightened ratio of non-high-density lipoprotein cholesterol (non-HDL-C) to HDL-C was associated with depression, with risk mediated by body mass index.

The authors wrote that their findings show a necessary update following early data from the Women’s Health Initiative.
Influenza vaccination provided direct, non–infection-based benefits in older adults.

Further research is needed to better understand which characteristics may better predict who are more likely to benefit from estrogen-based menopausal hormonal therapy (MHT) for anxiety.

Samantha Picking, PharmD, highlights pharmacists’ leadership in immunization initiatives, public health collaborations, and the evolving role of community pharmacy in preventive care.

If approved, SYD-101 would have been the first FDA-approved treatment for pediatric myopia, offering hope to millions of affected children in the US.