
Olezarsen effectively lowers triglycerides and enhances cardiovascular health, especially when combined with fibrates, benefiting pharmacists and patients alike.

Olezarsen effectively lowers triglycerides and enhances cardiovascular health, especially when combined with fibrates, benefiting pharmacists and patients alike.
New research reveals significant nonrespiratory complications in hospitalized children with influenza, highlighting the need for improved clinical awareness and antiviral treatment.
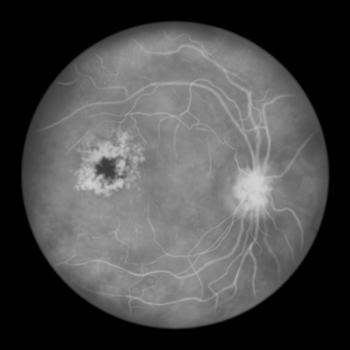
FDA approves an 8-mg aflibercept injection for macular edema following retinal vein occlusion (RVO), offering flexible dosing options for improved patient care.


Counsel patients about effective remedies for pediatric colds, sore throats, and safe OTC options for adults with hypertension to alleviate symptoms.

Proactive Monitoring and Patient Education Are Key Roles for Pharmacists

Pharmacists Can Educate Patients About Symptoms of Menopause, Associated Chronic Conditions, and Management Options
CDC's recent statement raises concerns about vaccine safety and autism links, igniting debate among health experts and fueling vaccine hesitancy.

Dexcom Smart Basal optimizes basal insulin dosing for type 2 diabetes, enhancing management with personalized recommendations and a 15-day CGM sensor.

The findings from the BATURA clinical trial were presented at the 2025 ACAAI Annual Scientific Meeting.

A recent study reveals diverse long COVID trajectories, highlighting the need for further research to understand symptoms and recovery variability.

New triglyceride-lowering therapy Olezarsen significantly reduces severe hypertriglyceridemia and acute pancreatitis risk, offering hope for patients.
New RSV vaccines for older adults enhance prevention efforts, yet awareness and uptake remain low. Pharmacists play a crucial role in improving vaccination rates.

Natural strategies and OTC options can help manage menopause symptoms, from hot flashes to mood changes, for a smoother transition.
The investigational, oral PCSK9 inhibitor has the potential to significantly reduce low-density lipoprotein cholesterol (LDL-C) in patients with hypercholesterolemia or patients with heterozygous familial hypercholesterolemia.

Measles resurges globally, threatening U.S. elimination status; pharmacists play a crucial role in vaccination efforts and outbreak response.
An evaluation of the different dosing and administration regimens of high-dose nitroglycerin for sympathetic crashing acute pulmonary edema.

Guselkumab and paltusotine received FDA approval, Kenvue announced the new Tylenol Precise Pain-Relieving Patch, and a new generic version of mifepristone received the green light from the FDA.

Depemokimab shows significant improvements in asthma symptoms and peak expiratory flow, supporting its twice-yearly use for severe asthma management.

Elinzanetant Showed Smaller Reductions in Bone Mineral Density Than Placebo
Plozasiran gains FDA approval to significantly lower triglycerides in adults with familial chylomicronemia syndrome, enhancing heart health management.
Amoxicillin is a favorable choice for outpatient use in pediatric patients given its relatively narrow spectrum of activity and palatable formulation.

An expert highlights the need for early detection of aortic stenosis symptoms in elderly patients, urging pharmacists and clinicians to monitor subtle changes.

Biykem Bozkurt, MD, PhD, FACC, highlights the need for reliable activity monitoring in heart failure care, addressing challenges of digital wearables in clinical practice.

The Annual Meeting Highlighted Emerging Research on Hormone Therapy, Brain and Heart Health, Disparities in Menopause Care, and the Expanding Role of Pharmacists

FDA Approval Introduces a First-in-Class Neurokinin Receptor Agonist Shown to Reduce Vasomotor Symptom Frequency and Improve Sleep Quality in Postmenopausal Women

New research reveals GLP-1 receptor agonists like semaglutide offer heart failure benefits beyond weight loss, emphasizing pharmacists' crucial role in patient care.

Pharmacists play a crucial role in holistic healthcare, enhancing patient care through education and collaboration with allied health professionals.

The SmartPhrase listed indications for PCV20 aligned with CDC guidelines and was incorporated into provider documentation, significantly improving vaccine uptake.