The approval is supported by data from a global phase 3 trial assessing the use of the drug for individuals with previously untreated IDH1-mutated acute myeloid leukemia.

The approval is supported by data from a global phase 3 trial assessing the use of the drug for individuals with previously untreated IDH1-mutated acute myeloid leukemia.

Karen Fancher, PharmD, BCOP, a member of the Patient Advisory Panel at the Hematology/Oncology Pharmacy Association (HOPA), explains how her experience as a patient with cancer and an oncology pharmacy specialist informs her work on HOPA’s Patient Advisory Panel.

Byron Yoshino, PharmD, CEO of Pharmacare Hawaii, discusses the future of the home infusion space within the United States in the coming years.

Dong Xu, PhD, MS, curators' distinguished professor at the University of Missouri College of Engineering, discusses how artificial intelligence is being used to develop new drug therapies for medical treatments targeting cancers and other diseases.

Byron Yoshino, PharmD, CEO of Pharmacare Hawaii, discusses the rapidly growing home infusion space within Pharmacare Hawaii’s network of pharmacies.

Kirollos Hanna, PharmD, BCPS, BCOP, discussed how PARP inhibitors have changed the treatment landscape of ovarian cancer.
Watch for alopecia, arthralgia, diarrhea, fatigue, nausea, pyrexia, rash, and vomiting, among others.
Xofigo is indicated for the treatment of patients with castration-resistant prostate cancer, symptomatic bone metastases, and no known visceral metastatic disease.

Study results also show a 50% increase in the precancerous condition, Barrett esophagus, between 2012 and 2019, in the same population.
Experts discuss the role of pharmacists in improving screening rates for breast cancer, how they can support patients, and improve outcomes.
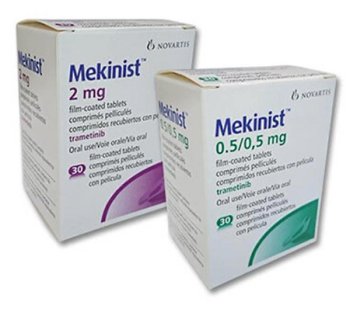
Trametinib (Mekinist) is a kinase inhibitor indicated as a single agent for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations as detected by an FDA-approved test.

Latest results of the phase 3 IKEMA trial demonstrate the longest median progression-free survival on a proteasome inhibitor backbone in this population, Sanofi says.
Independent committee recommends the phase 3 clinical trial continues to assess other primary and secondary endpoints for the treatment of unresectable or metastatic urothelial carcinoma.
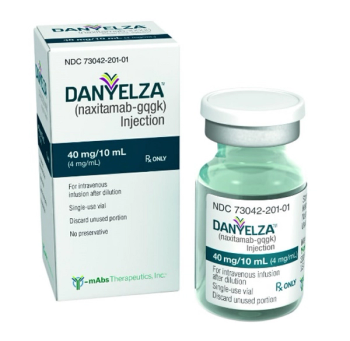
Naxitamab-gqgk (Danyelza) is for the treatment of pediatric and adult patients with relapsed or refractory high-risk neuroblastoma in the bone or bone marrow who have demonstrated a partial response, minor response, or stable disease to prior therapy.

Ribociclib plus fulvestrant achieved a median overall survival of 67.6 months in the first line setting for postmenopausal women with HR-positive/HER2-negative advanced breast cancer.
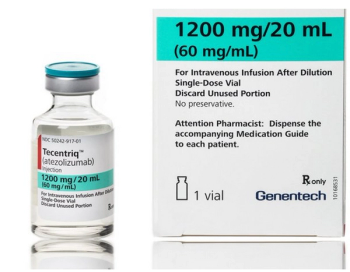
Atezolizumab (Tecentriq) is indicated for use in urothelial carcinoma, non-small cell lung cancer, triple-negative breast cancer, small cell lung cancer, hepatocellular carcinoma, and melanoma.

Aklilu Tedla, vice president of strategy and business development at Cardinal Health, discusses the cell and gene therapies in the pipeline for approval by the FDA.

Aklilu Tedla, vice president of strategy and business development at Cardinal Health, discusses the future of pharmacies as more cell and gene therapy products become available to patients.

Repotrectinib awarded breakthrough therapy designation for patients with ROS1-positive metastatic non–small cell lung cancer previously treated with a ROS1 tyrosine kinase inhibitor and who were not previously administered platinum-based chemotherapy.
The SKYSCRAPER-01 trial evaluating the drug plus Tecentriq demonstrates that it did not meet its co-primary endpoint of progression-free survival.

Joel Wayment, vice president of operations for 3PL Services at Cardinal Health, discusses how the pharmaceutical supply chain currently manages the transport of CAR T products to sites of administration.

Ray Tancredi, divisional vice president of Specialty Pharmacy, Development & Brand Rx/Vaccine Purchasing at Walgreens, discusses blockbuster drugs in the oncology space that have been approved this year or may be hitting the market soon.

Tracy Russell, senior director, State Government Affairs at CoverMyMeds, discusses state and federal legislation that may be impacting the future of specialty pharmacies and the patient care they deliver.

First phase 3 trial in this setting shows improved overall survival rates with an immunotherapy added to chemotherapy over the standard-of-care chemotherapy alone, company says.

In an interview with Pharmacy Times, Amy Pfeifer, PharmD, BCPS, CSP, of AllianceRx Walgreens Prime, discussed the important role that pharmacists can play in the treatment of prostate cancer.

Jelmyto is an alkylating drug indicated for the treatment of adult patients with low-grade upper tract urothelial cancer.
Palbociclib is indicated for adults in combination with an aromatase inhibitor as initial endocrine-based therapy in postmenopausal women or in men.

The drug combination achieved a median overall survival of more than 5 1/2 years in the first-line setting for postmenopausal women with HR+/HER2- aBC1, the company says.
Obinutuzumab is a CD20-directed cytolytic antibody used to treat certain types of blood cancer.

Trastuzumab deruxtecan (Enhertu) approved for adult patients with unresectable or metastatic HER2-positive breast cancer who were previously administered an anti–HER2-based regimen.