
Additionally, the safety profile in DESTINY-Lung01 was consistent with previous clinical trials with no new safety concerns identified.

Additionally, the safety profile in DESTINY-Lung01 was consistent with previous clinical trials with no new safety concerns identified.
Session highlights novel agents that have recently entered, or will imminently enter, into phase 1 clinical trials.
The oral PARP inhibitor is associated with increased PFS, regardless of BRCA mutational status.

Medicaid expansion implementation was associated with a decrease in the number of uninsured patients from 6.7% pre-expansion to 3.6% post-expansion.

Elaine Jaffe, MD, National Institutes of Health (NIH) distinguished investigator at the National Cancer Institute at NIH, discusses points of note in the evolution of lymphoma classification during her clinical and investigational research on the subject.

Sonia Oskouei, PharmD, vice president of biosimilars at Cardinal Health, on the results of Cardinal Health’s assessment of the current biosimilars market published in the 2022 Biosimilars Report.
David DeRemer, PharmD, BCOP, FCCP, FHOPA, a past-president on the board of Hematology/Oncology Pharmacy Association (HOPA), discusses why a greater focus on colorectal cancer screening was important to highlight at the HOPA 2022 annual conference.

Bevacizumab-maly (Almysys; mAbxience) is the third biosimilar of bevacizumab (Avastin) approved in the United States.

New therapies and combination regimens may allow a more tailored approach.

David DeRemer, PharmD, BCOP, FCCP, FHOPA, a past-president on the board of Hematology/Oncology Pharmacy Association (HOPA), discusses key takeaways from the HOPA 2022 annual conference.

The companies have decided to unblind the trial and perform no additional analyses for the OS endpoint because there was no clinical benefit observed in the doublet therapy arm.
The findings, published the New England Journal of Medicine, showed treatment with rilzabrutinib led to a rapid, durable increase in platelet count while supporting an acceptable safety profile.

In the first episode of Pharmacy Focus: Oncology Edition, we spoke with Ron Lanton, JD, principal at Lanton Law.
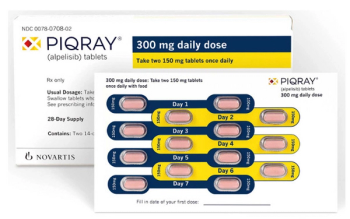
Piqray is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with HR-positive/HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer, as detected by an FDA-approved test following progression on or after an endocrine-based regimen.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses how experience as a caregiver can help inform the work of an oncology pharmacist on the clinical care team.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses how oncology pharmacists with experience as a caregiver can utilize those skills while still honoring the boundaries of both themselves and their patients.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses some of the unique struggles that come from being an oncology pharmacist and a caregiver.

Sarah Wheeler, PharmD, BCOP, clinical pharmacy specialist in Hematology/Oncology at UF Health Shands Cancer Hospital, discusses how oncology pharmacists can help their patients overcome some of the barriers patients with cancer may encounter during treatment.

Benyam Muluneh, PharmD, BCOP, CPP, assistant professor at the University of North Carolina Eshelman School of Pharmacy, discusses his presentation at the HOPA 2022 annual conference on the Oral Chemotherapy Collaborative (OCC) and how it works to optimize care for patients taking oral anti-cancer agents.
Vusolimogene oderparepvec is in development for the treatment of multiple skin cancers either alone or in combination with anti-PD1 therapy.

The investigational selective, covalent, and orally bioavailable KRASG12C¬ inhibitor shows a 57% confirmed overall response rate at the recommended dose of 200 mg twice daily.
The FDA previously granted Fast Track Designation for the combination of lifileucel and pembrolizumab in metastatic melanoma.

Based on event-free survival and pathologic complete response results, the FDA approved nivolumab in combination with platinum-doublet chemotherapy in March 2022.
Results show that 64% of individuals treated with lorlatinib were without disease progression after 3 years compared with about 19% of those treated with crizotinib (Xalkori).

Numbers have remained below prepandemic levels.

Blue Earth Therapeutics will begin an open-label, multicenter trial to evaluate a next-generation radiopharmaceutical for metastatic castration-resistant prostate cancer.
Clinical data from phase 1/2 LIBRETTO trial show evidence of meaningful clinical outcomes for patients, including those with difficult-to-treat brain metastases.
Sotorasib demonstrates clinical benefit and prolonged tumor response seen, with a 40.7% objective response rate.

Biosimilars could save consumers more than $100 billion in the coming years.

Britny Brown, PharmD, BCOP, vice chair of the Hematology/Oncology Pharmacy Association (HOPA) DEI Task Force and clinical assistant professor at the University of Rhode Island College of Pharmacy, discusses the role of the task force within HOPA and its impact on the field more broadly.