
Kelly McAuliff, PharmD, BCOP, CSP, spoke about research into the impacts of the COVID-19 pandemic on oral oncolytic adherence, particularly for patients with ovarian cancer, prostate cancer, or chronic myelogenous leukemia.

Kelly McAuliff, PharmD, BCOP, CSP, spoke about research into the impacts of the COVID-19 pandemic on oral oncolytic adherence, particularly for patients with ovarian cancer, prostate cancer, or chronic myelogenous leukemia.

Both patients and providers should recognize the benefits of early referrals to palliative care.

Ahmed Kotb, MD, vice president of US medical affairs, oncology, at AbbVie, discussed new findings from the REFINE trial and what they could mean for patients with myelofibrosis.

With several recent approvals, new CAR T therapies are having significant impacts in multiple myeloma treatment.
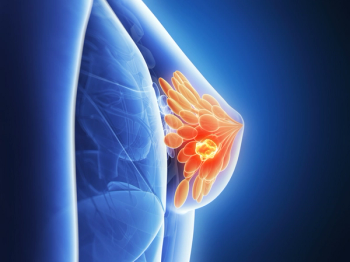
With a median follow-up of 90 months, individuals receiving palbociclib in combination with letrozole had numerically longer OS compared to placebo, but it was not deemed statistically significant.

Overall outcomes were consistent with axicabtagene ciloleucel in the real-world setting, regardless of race or ethnicity, in adults with relapsed or refractory large B-cell lymphoma.
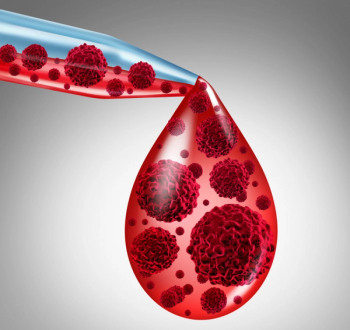
Brentuximab vedotin (Adcetris) plus doxorubicin, vinblastine, and dacarbazine shows improved overall survival compared with standard chemotherapy in patients with previously untreated, advanced stage classical Hodgkin lymphoma.

An immune checkpoint inhibitor plus ramucirumab found to improve overall survival versus standard of care in patients with advanced non-small cell lung cancer.

Ibrutinib plus bendamustine-rituximab and rituximab maintenance was found to lower the risk of disease progression or death by 25% compared with placebo in patients 65 years of age or older with newly diagnosed mantle cell lymphoma.

Uliledlimab plus toripalimab (Tuoyi) shows significant response rates in patients with advanced non–small cell lung cancer previously ineligible to receive standard-of-care treatment.

The efforts of pharmacists remain critical to the success of treatment outcomes.

Analysis shows that different genetic mutation profiles in tumors harboring PIK3CA mutation did not affect the treatment benefit with alpelisib (Piqray) combined with fulvestrant in individuals with HR+/HER2- advanced or metastatic breast cancer.

Improvement in recurrence-free survival was seen primarily in individuals with very high-risk renal cell carcinoma, whereas individuals with intermediate high-risk disease showed no improvements in survival.

The median overall survival was 66 months for individuals with hormone receptor-positive/human epidermal growth factor receptor-negative advanced or metastatic breast cancer administered at least 1 dose reduction of ribociclib (Kisqali) from the starting dose of 600 mg.
Oncology pharmacists can help ensure patient access to lifesaving therapies.

The FDA approved decitabine/cedazuridine in 2020 for treatment of adults with myelodysplastic syndromes.

Ogi Kavazovic, co-founder and CEO of House Rx, discusses the company's technology platform and pharmacy service and how it can better enable community oncology pharmacy practices to offer medically integrated dispensing.
Company releases data at the American Society of Clinical Oncology Annual Meeting 2022 showing that the drug is well-tolerated in patients with solid tumors.

There are a huge number of medical facilities in Ukraine that run international clinical trials, with the FDA noting that more than 250 drugs and devices were undergoing clinical research in Ukraine.
The new study analyzed KSHV’s latent-lytic switch, a process in which the virus exits its dormancy state to replicate in the host cell.

Pegfilgrastim-pbbk is the third oncology biosimilar developed by Amneal to gain FDA approval this year.

Tesh Khullar, co-founder and president of House Rx, discusses how social determinants of health can impact time to treatment initiation.

Analysis also shows a reduction in the need for opiate painkillers, and there was a decrease in other cancer-related symptoms, according to results published in Frontiers in Pain Research.

Pharmacy Times will be covering the American Society of Clinical Oncology (ASCO) 2022 Annual Meeting live in Chicago, Illinois, from June 3 through 7, 2022.
Tisagenlecleucel was shown to be effective in high-risk patients, including those who were heavily pretreated or had refractory disease.
We spoke with Kelly McAuliff, PharmD, BCOP, CSP, to learn about data being presented at the ASCO 2022 Annual Meeting.

Nivolumab (Opdivo) with fluoropyrimidine- and platinum-containing chemotherapy and nivolumab plus ipilimumab (Yervoy) approved as first-line treatments for unresectable advanced or metastatic esophageal squamous cell carcinoma.

Further sensitivity analyses to control for family alcohol consumption, family history, liver disease, and smoking, finds no convincing evidence that these factors affected the results.
Taking multiple prescription drugs and supplements can be especially dangerous for individuals with cancer who are about to undergo therapy.

Treatment with nirogacestat resulted in a 71% reduction in the risk of disease progression among adults with progressing desmoid tumors.