
The results showed a 38.1% confirmed objective response rate among HER2-positive patients who received tucatinib in combination with trastuzumab.

The results showed a 38.1% confirmed objective response rate among HER2-positive patients who received tucatinib in combination with trastuzumab.
Poziotinib is an irreversible pan HER2 inhibitor with activity against mutations of HER1, HER2, and HER4.
Approximately one-third of the individuals had an intracranial response among those with central nervous system metastases.

Dronabinol (Syndros) is a cannabinoid indicated in adults for the treatment of: anorexia associated with weight loss in patients with AIDS and nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments.

Patients at highest risk of cancer recurrence, including people with lymph node-positive disease, saw the greatest reduction in the risk of recurrence or death with the pertuzumab-based regimen

Organizations such as the Appalachian Community Cancer Alliance are working to leverage technology and improve barriers to care.
Treatment's approval marks exciting new development for patients with ES-SCLC to prevent CIM.

This month, we spoke with Bob Luschen, PharmD, clinical coordinator for the pharmacy department at cancer Treatment Centers of America, Atlanta.

Cole McCoy, PharmD, considers if maintenance therapy is standard of care and what is involved with appropriate transitions upon discharge.

Cole McCoy, PharmD, details his experiences with oral maintenance therapies, specifically oral azacitidine.
About one-third of participants in the phase 1b cohort of the KRYSTAL-1 study show an intracranial response, according to Mirati Therapeutics.

The nature of CLL being a long-term, incurable disease makes its treatment a challenge as it is more focused toward improving patient’s quality of life.

With the rapid pace of change in biosimilar payer benefit design, efforts to stay regularly updated on policy changes ahead of time become critical for patient care.

Experts discuss the pros and cons of using oral azacytidine to treat patients with acute myeloid leukemia.

With a lack of head-to-head studies comparing combination regimens in frontline metastatic renal cell carcinoma, deciding on how to choose the right regimen can pose a challenge.

Crizotinib has also been approved to treat non-small cell lung cancer that has spread to other parts of the body and is caused by a defect in either the ALK or ROS1 gene.

Pursuing the expanded access process for treatment may be the best option for patients who have exhausted all available FDA-approved drugs and clinical trials.

Dutch biopharmaceutical company Byondis submitted the BLA for [vic-]trastuzumab duocarmazine (SYD985) in the treatment of patients with HER2-positive metastatic breast cancer with the goal of improving patient outcomes.

Some anti-abortion laws may lead to legal action against health care providers who prescribe or provide medications that can be used to induce abortions.

From the first approved CAR T therapy for multiple myeloma, to the recently-approved ciltacabtagene autoleucel, significant steps have been taken.

The FDA has approved fam-trastuzumab deruxtecan-nxki (Enhertu) for previously treated HER2-positive breast cancer and gastric or gastroesophageal junction adenocarcinoma.

Experts discuss tools and trainings that health care institutions have established during the pandemic to support hematology-oncology pharmacists who are struggling with wellness and mental health challenges.
Screening participation increased over time, but the increase was smallest among individuals aged 50 to 54.

An intelligence-based system could help improve the timeliness of referrals to palliative care and hospice services in a community oncology environment.
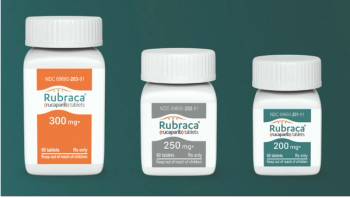
Rucaparib (Rubraca) is a poly (ADP-ribose) polymerase (PARP) inhibitor used for ovarian cancer, fallopian tube cancer, primary peritoneal cancer, and castration-resistant prostate cancer.

James McCloskey, MD, analyzes result data from the QUAZAR AML-001 trial that focused on oral azacitidine.

Cole McCoy, PharmD, and Danielle Marcotulli, APN, RN, MSN, FNP-BC, AOCNP explain the additional agents being studied as maintenance therapy options.
Christopher Fine, MD, FACC, a cardiologist at National Jewish Health, discusses how pharmacists can support patients with cancer who have a much higher risk of heart disease.

By using technology solutions, pharmacists can be further integrated into the care team.
Christine Walko, PharmD, BCOP, FCCP, discusses some new developments for PARP inhibitors in cancer care for patients with homologous recombination deficiency.