The candidate has already demonstrated anti-tumor activity in chemotherapy-naïve patients.

The candidate has already demonstrated anti-tumor activity in chemotherapy-naïve patients.
A risk-stratification screening approach that considers screening at an earlier age based on risk factors might increase the likelihood of catching the cancer before it turns symptomatic.
The MRD-guided treatment approach has a significant advantage over chemotherapy.

Compared with chemotherapy, nivolumab plus ipilimumab reduced the risk of disease progression or death by 79%, but further research is needed to assess overall survival.
A recent study sheds light on an alarming number of hormone-disrupting chemicals that are found in everyday settings.

It can also help identify patients who would benefit from disease-specific therapies.
Data support the quadruplet regimen as a new standard of care for transplant-eligible patients.
This RNA type might act as an onco-suppressor, and higher levels may indicate better prognosis.

Berberine was associated with a reduction in inflammatory markers in the lungs, which was caused by the colorectal cancer.
The IQVIA Global Use of Medicines report for 2024 predicts that loss of brand exclusivity will negatively impact global market growth.
There is a significant efficacy-effectiveness gap in standard-of-care regimens.

The third edition of the Pharmacy Quality Alliance resource guide includes 40 total SDOH services and 8 new services, as well as 10 initiative updates from the previous 2 editions.

Further policy changes to promote gender equity may be needed.

The treatment is for patients with KRAS G12C-mutant metastatic NSCLC who have previously received at least 1 systemic therapy and were not treated with a KRAS G12C inhibitor.
Ph-like ALL may be predominantly prevalent in Hispanic and Latino populations.
PTX-252 is a novel molecular drug designed to increase cancer cells' sensitivity to chemotherapy in the treatment of acute myeloid leukemia.

Significant study results could inform clinical practice strategies.
Presentations detail clinical trial results and focus on drugs in the pipeline.
The approval was based on the results of a phase 3 trial that found an improvement in progression-free survival among individuals treated with pembrolizumab.

2023 was an impactful year for the field of oncology pharmacy.

Biosimilars are proven to be highly similar to the original products with no clinical meaningful differences.

In addition to the current principles and treatment regimens, there are upcoming data on myelofibrosis drugs that could be reported as early as 2024.

Not only were individuals with BMIs of 30 and higher likely to have MGUS, but those who reported heavy smoking and short sleep were also more likely to have detectable levels of MGUS.
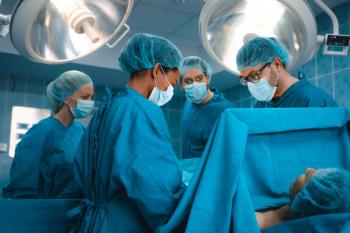
The separation between those who travel for care compared to those who go to the local hospital may drive inequalities in health care.
A majority of patients who received first-line chemotherapy switched to amivantamab-vmjw plus chemotherapy as second-line therapy.
A leukemia clinical pharmacy specialist delves into the significance of educating patients on treatments and serving as a resource for both patients and health care providers.
In addition, patients who were treated with liso-cel therapy had a higher average life expectancy and QALYs, compared to those who were treated with standard care.
The pharmacist can assist with managing drug interactions, patient education, therapy selection, physician education, and more.
Compared to standard of care TKIs, asciminib also presented favorable safety and tolerability profiles with fewer reported AEs among patients.

Individuals with ischemic stroke that resided in areas with an indoor radon concentration greater than 4 pCi/L had an increased risk of developing clonal hematopoiesis of indeterminate potential.