
A study that analyzed data from 2000 to 2021 indicated improvements for both leukemia-free survival and overall survival for older patients with acute myeloid leukemia.

A study that analyzed data from 2000 to 2021 indicated improvements for both leukemia-free survival and overall survival for older patients with acute myeloid leukemia.
Benefits throughout post-progression endpoints and outcomes were reported for osimertinib in combination with chemotherapy.
Early treatment administration with this regimen also promoted a faster decrease in viral load and shorter viral shedding in patients with hematologic diseases who were positive for COVID-19.

Evidence suggests foods that activate sirtuin proteins may be beneficial for metabolic syndromes
Pembrolizumab in combination with chemotherapy followed by maintenance olaparib did not meet its pre-specified statistical criteria of overall survival or progression free survival.

The podcast discusses how City of Hope Chicago fully integrates oncology pharmacists into care teams by positioning them in clinics, improving communication and collaboration to minimize delays for patients and enhance care delivery.
The orphan drug designation (ODD) is a result of positive findings from a phase 2 clinical trial which demonstrated a 100% clinical benefit rate in patients.
The test’s sensitivity in distinguishing colorectal cancer, in combination with real-world adherence, showed its ability to detect cancer at a curable stage.
PT886 (Phanes Therapeutics) received fast track designation 2 years after receiving orphan drug designation by the FDA.

Pharmacists can educate patients on current clinical evidence of cannabis’s therapeutic benefit, as well as cannabis’s complexities, risks, and potential adverse effects.
The treatment is indicated for patients with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancers.
Merck will also conduct clinical trials in both males and females to evaluate the efficacy of a single dose of their current human papillomavirus vaccine compared with the approved 3-dose regimen.
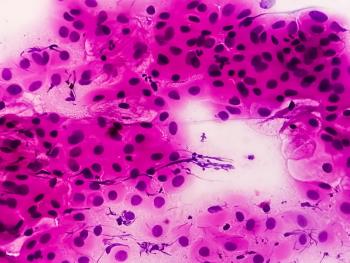
Methods of treating cervical intraepithelial neoplasia are limited and invasive. The need for better treatments is clear; however, there are several promising therapies under development.

Findings show that magnetic resonance imaging and lumbar puncture are not always needed when managing complications of CAR T-cell therapies, but may be beneficial in certain cases.

Previously, the study met the other primary endpoint of progression free survival, showing statistically significant and clinically meaningful improvements compared to chemoradiotherapy alone.

The approval comes after positive results from the PhALLCON study, however, further research is needed to confirm immature event-free survival findings.

The agency will focus on collaboration to protect public health; advancing regulatory approaches; developing standards, guidelines, and best practices; and supporting research that evaluates and monitors AI performance.

The authors note that understanding the DNA methylation profile in leukemia can help predict whether or not a patient will respond to treatment.

The decision to vote in favor of idecabtagene vicleucel comes after positive phase 3 trial results demonstrating its efficacy compared with standard regimens.

The FDA-approved topical and oral agent is currently indicated for adult patients with patterned alopecia.
Chimeric antigen receptor T-cell therapies were associated with higher incidences, grades of severity, and longer duration of cytokine release syndrome compared with bispecific antibodies.

In a phase 1/2 clinical trial, lisocabtagene maraleucel helped patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) achieve complete response rates.

Results from the phase 3 RATIONALE 302 trial showed tislelizumab-jsgr prolonged survival compared to chemotherapy in patients who received prior systemic treatment.

This finding was evident in both examined groups and regardless of the patients' B-cell maturation antigen-directed therapy status.
Furthermore, the study authors indicated that patients with lymphoma and community-acquired pneumonia have broader dysregulated responses.

The combination of GZ17-6.02 and bortezomib reduced of HDAC, and as a result ATG13 phosphorylation was enhanced, BAK levels increased, and BCL-XL levels were reduced.

Counsel patients about smoking cessation products

This initiative aims to raise worldwide awareness of the disease while trying to strengthen the connection between members of the multiple myeloma community.
The study results also indicated that graft versus host disease status was associated with non-compliance following bone marrow transplantation.
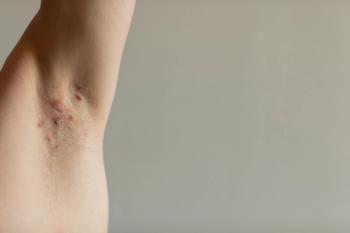
In addition, the length of hospital admissions were greater in patients with hidradenitis suppurativa-associated cutaneous squamous cell carcinoma compared to other admissions.