
The risk of developing lymphoma remained nearly the same between patients with Crohn disease and ulcerative colitis.

The risk of developing lymphoma remained nearly the same between patients with Crohn disease and ulcerative colitis.

This model, which uses technology that discovers black holes, pulls from real data to evaluate cancer treatments, optimizing treatment choices for patients with different cancers.

Liz Vogel, CPhT-Adv, CSPT, discusses the procedures and best practices for safe compounding of hazardous and non-hazardous drugs.
The accelerated approval comes after 48-week major molecular response rate data.
The INTerpath-009 trial assessed a combination of V940 with pembrolizumab among individuals with stage II, IIIA, IIIB (N2) non–small cell lung cancer.
The investigators note that these findings are significant because bile duct cancer is a rare disease with limited treatments and low survival rates.

The new indications include pediatric individuals with acute lymphoblastic leukemia and polyarticular juvenile idiopathic arthritis.

Apalutamide is a hormone therapy indicated for men with nonmetastatic castration-resistant prostate cancer (nmCRPC).

Expert discussed the potential of pelareorep, a novel immunotherapeutic agent, in treating advanced or metastatic breast cancer.

NCCN recommends ribociclib as a preferred CDK4/6 inhibitor adjuvant therapy for patients with HR+/HER2- EBC in combination with an aromatase inhibitor.

Nathan Vanderford, PhD, MBA speaks about barriers to cancer care access experienced by patients in rural communities.

Sara Rogers, PharmD, discusses the formation and goals of the Standardizing Laboratory Practices in Pharmacogenomics (STRIPE) initiative, which seeks to standardize pharmacogenomic practices and improve clinical decision-making by establishing consensus across all stakeholders.

Their expertise complements multidisciplinary teams in a variety of clinical settings.

Ryan Nelson, PharmD, discusses the STRIPE Annual Meeting and Consensus Workshop’s focus on unifying pharmacogenetic guidelines across major organizations, such as the FDA, European Medicines Agency, Clinical Pharmacogenetics Implementation Consortium, and National Comprehensive Cancer Network.

Despite self-reported improvements in cognitive function, neuropsychological tests showed little differences between 2 patient groups.

Stephanie White, PharmD, CSP, is presenting at the NCODA Fall Summit in Orlando, Florida.
The adjuvant therapy has demonstrated varied efficacy for patients.
These therapies are being investigated in earlier lines, with several new treatments in development.

Benjamin Brown of Standardizing Laboratory Practices in Pharmacogenomics (STRIPE) discusses his own experience as a patient with cancer and how consensus around pharmacogenomic practices can improve the lives of patients.
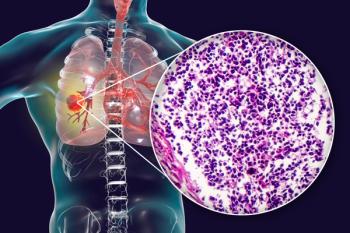
An overview of current FDA-approved ROS1-targeted therapies.

Ginger Blackmon, PharmD, will discuss enhancing patient cancer care through implementation of medically integrated oncology team and NCODA’s PQI resources.

Pharmacy Times will be at the NCODA Fall Summit in Orlando from October 23 to October 25, 2024.

Dispensing, communicating, and recording information may look different at each center.

Endometriosis is a systemic disease that can affect multiple parts of the body, not just the pelvis.
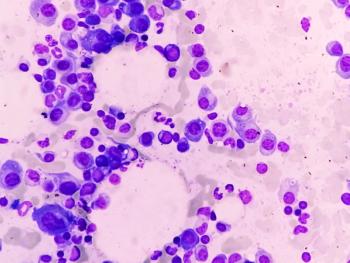
Comprising most cases of this rare form of lymphoma, it is essential that pharmacists and treatment providers are aware of the health-related burdens patients with these subtypes consistently face.
The therapy offers new hope in solid tumor treatment with fewer adverse effects.
There are gonadotoxic risks of chemotherapy, radiation, and surgery that are important to discuss with patients.
Multiple P13K inhibitors have showed promise in treating genetically-mutated forms of breast cancer.
The study was conducted on a small subgroup of Japanese patients with myelofibrosis.
The diagnostic test is a companion to vorasidenib, an isocitrate dehydrogenase inhibitor that received FDA approval this past summer.