
While waiting for budgetary approvals, there are some simple initiatives outpatient oncology practices can initiate that yield high rewards and quick wins yet cost little more than an investment of time.

While waiting for budgetary approvals, there are some simple initiatives outpatient oncology practices can initiate that yield high rewards and quick wins yet cost little more than an investment of time.

Invikafusp alfa (STAR0602; Marengo Therapeutics Inc) is being investigated as a treatment for advanced colorectal cancer with high tumor mutational burden.
In a phase 2 trial (NCT05400226), abenacianine helps surgeons visualize tumors in lung during surgery and is safe and well-tolerated in patients.
Serplulimab plus HLX04 demonstrates a favorable safety profile in patients with advanced hepatocellular carcinoma.

The late former president played a critical role in advancing cancer care, as well as efforts to eradicate preventable diseases.
For patients with multiple myeloma who may experience an infection while receiving teclistamab, supplementation with intravenous immunoglobulin (IVIG) can help resolve and prevent serious complications.
The new designation for the selective PPAR⍺ antagonist follows positive phase 1b/2 clinical trial results.

Palbociclib demonstrates safety and efficacy in combination with anti-HER2 and endocrine therapies.

The advisory emphasizes the need for a comprehensive approach to reducing alcohol-related cancer by combining prevention, education, and treatment to improve public health outcomes.

AI is revolutionizing oncology drug development, enhancing therapeutic identification and reducing approval times, but use of this tool must be carefully implemented to mitigate risks and ensure regulatory compliance.
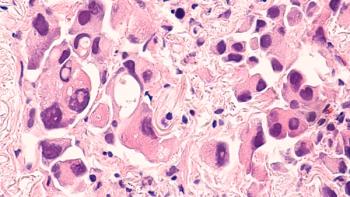
Sunvozertinib was originally approved in China, making it the world’s first and only oral treatment for these patients.

A pooled analysis showed regular consumption of coffee and tea was associated with decreased risk of certain cancers.
If approved, the combination of avutometinib with defactinib could be the first FDA-approved treatment option for low-grade serous ovarian cancer.
Calcipotriol in combination with 5-FU triggers Th2 immunity to prevent skin cancer.
The analysis shows patients achieved deep and durable responses despite being ineligible for the CARTITUDE-1 trial.
Pharmacists should be aware of the impacts that anemia in patients being treated for cancer has on daily activities and overall quality of life.

Compliance is achievable for outpatient oncology practices with careful planning and execution.
Patients with hormone receptor–positive and human epidermal growth factor receptor 2 breast cancer are particularly at risk.
Patients with relapsed, refractory disease achieved deep, durable responses with equecabtagene autoleucel.
The model, Deep-IO, shows improved specificity and sensitivity compared with existing predictive biomarkers.

Treatment options may include treating the anemia with blood transfusions, androgen therapy, thalidomide, and other medications.

Tislelizumab is approved in combination with platinum and fluoropyrimidine-based chemotherapy as first-line treatment of unresectable or metastatic HER2-negative tumors.
The approval offers patients with solid tumors an alternative to intravenous PD-1 inhibitor administration.

The potential treatments include ivermectin, methylene blue, glucagon-like peptide-1 receptor agonists, and low-dose naltrexone.
Constantine Tam, MD, discusses zanubrutinib's efficacy in CLL/SLL, emphasizing its durability, high response rates, safety considerations, and evolving therapeutic potential in combination with other agents.
Bispecific antibodies amivantamab and tarlatamab hold promise for patients.
Anthony Perissinotti, PharmD, BCOP, discusses unmet needs and trends in managing chronic lymphocytic leukemia.

The program develops patient-centered oncology pharmacists through comprehensive training.

Yi Lin, MD, PhD, highlights the importance of achieving MRD negativity in multiple myeloma, CAR T-cell therapy outcomes, and the critical role of pharmacists in patient care.
Here is an updated overview of the role of BCMA-directed therapies following the 2024 ASCO Annual Meeting and EHA Congress.