Gecacitinib Demonstrates Safety, Efficacy Over Hydroxyurea in Patients With Myelofibrosis and Anemia
Gecacitinib is a novel inhibitor targeting both JAK and ACVR1.

Gecacitinib is a novel inhibitor targeting both JAK and ACVR1.
This study evaluated factors affecting the duration of the infusion appointment times for 392 clinical trial patients.

Of these chemicals, 414 were found to be commonly used in plastics and consumer goods, such as food packaging and personal care products.

Pharmacists bring a unique perspective to health care that bridges clinical knowledge, patient-centered care, and operational expertise.

Pharmacy Times attended the 2024 San Antonio Breast Cancer Symposium from December 10 to December 13.
The treatment landscape for multiple myeloma continues to evolve.
A study in Lancet Haematology revealed that minority ethnic patients face significantly poorer outcomes after hematopoietic cell transplantation compared to White patients.

Pharmacists identify appropriate patients for new oral and antibody drug conjugate treatments and provide counseling to help patients navigate the evolving treatment landscape.
This case highlights the importance of recognizing ICPI-AKI, implementing effective therapeutic interventions, and understanding the clinical context to prevent irreversible renal damage.
The indication is for patients with locally advanced or metastatic disease who have not previously received treatment with an ALK-inhibitor.
The researchers suggest that an imbalance between pro-inflammatory and resolving lipid mediators may drive tumor growth.

The radiation emerging from these isotopes can be used in 2 different ways in cancer trials: imaging (detection of tumors) and therapy (destruction of tumors).

Firas El Chaer, MD, discusses newly-presented data at ASH demonstrating nuvisertib's effectiveness in myelofibrosis.
Abstract data presented in September demonstrate that the drug elicited strong antitumor activity in the second line in both platinum-sensitive and -resistant patients.

Obesity is associated with increased distant recurrence and breast cancer mortality in all types of early-stage breast cancer.

The indication is for adults with metastatic cutaneous squamous cell carcinoma (mCSCC) or locally advanced CSCC (laCSCC) who are not candidates for curative surgery or radiation.
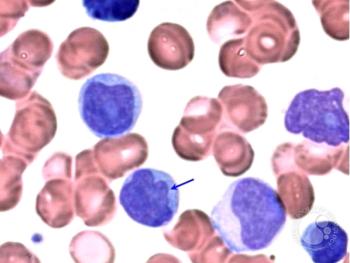
Alison Moskowitz, MD, discusses the clinical considerations, dosing strategies, and the critical role of pharmacists in managing toxicities and tailoring treatment plans for dual-targeted therapy with ruxolitinib and duvelisib.

AI-powered analysis of mammography images can identify women at high long-term risk of breast cancer, enabling targeted prevention strategies.

Adding anthracyclines to chemotherapy may improve survival in high-risk breast cancer patients.
Improvements were observed regardless of whether patients initiated ruxolitinib in the second or third line of treatment.
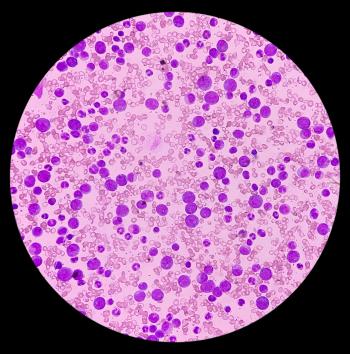
Compared with placebo, patients with relapsed/refractory (R/R) follicular lymphoma (FL) who were treated with tafasitamab showed a median progression-free survival (PFS) of 22.4 months.
Ira Zackon, MD, explains where future research lies regarding the implementation of bispecific antibodies in community oncology settings.

The novel camizestrant-ribociclib combination demonstrated encouraging data in advanced, pre-treated ER+/HER2- breast cancer.

Breast cancer prevention vaccines could have significant positive societal impact by reducing cancer diagnoses.
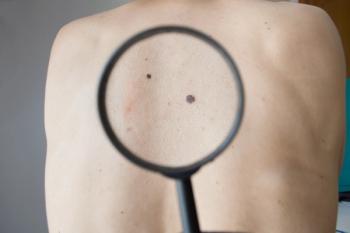
Currently, the treatment is undergoing evaluation in a phase 2a trial.

This update follows May 2021 guidelines which recommended patients with primary biliary cholangitis (PBC) with advanced cirrhosis discontinue obeticholic acid.

The combination of elacestrant and abemaciclib showed promising 8.7-month progression-free survival in ER+, HER2- advanced breast cancer, including in patients with ESR1 mutations.

Patients with chronic graft-versus-host disease (cGVHD) were most likely to receive belumosudil in the fourth line (33.7%) setting or the fifth through seventh line (33.1%).

The assessment of tumor-infiltrating lymphocytes can provide valuable insights to guide personalized treatment decisions for breast cancer patients.

Both risk-reducing mastectomy and salpingo-oophorectomy were associated with significant improvements among young BRCA carriers with breast cancer.