Presentations detail clinical trial results and drugs in the pipeline.

Presentations detail clinical trial results and drugs in the pipeline.
Palbociclib is an oral CDK4/6 inhibitor that was approved by the FDA in 2017.

The report from the Office of Inspector General highlights inconsistencies, lack of transparency, and the need for stronger safeguards.

Compared with chemotherapy, Dato-DXd demonstrates favorable median progression-free survival and overall survival in a phase 3 clinical trial.

The FDA proposes to cap the nicotine level at 0.7 mg per gram of tobacco in cigarettes and certain other combusted tobacco products.
The authors note that future research will observe personality changes when psilocybin is used in patients with opioid-use disorder.

These drugs are set to revolutionize their respective disease states in 2025.
Data from the CodeBreaK 300 trial supported the FDA's approval.
Acalabrutinib with bendamustine and rituximab shows efficacy in patients with untreated mantel cell lymphoma by increasing progression-free survival in the ECHO trial.
As per the Inflation Reduction Act, the negotiations with the drug companies of the 15 drugs will occur in 2025, with the negotiated prices going into effect in 2027.
Hormonal contraceptives did not increase breast cancer risk in patients with BRCA2 mutations.
The combination yields promising results but was associated with high incidence of toxicity and infection.
However, investigators also find that the combination did not meet the primary end point of increasing progression free survival.


Judith Alberto, MHA, RPh, BCOP, director of clinical initiatives at Community Oncology Alliance, discusses key policy issues affecting community oncology pharmacists in 2025.
Studies shows that Red Dye No. 3 was associated with tumor growth in male rats.

Radon is a tasteless, odorless radioactive gas associated with DNA damage and high genomic tumor instability.
Patients with methotrexate (MTX)-induced acute kidney injury treated with glucarpidase were 2.70-times more likely to have kidney recovery and recover faster.
The approach favors administration of frequent low-dose chemotherapy to reduce toxicities and optimize outcomes.

Building on a previous report that revealed corrupt business practices among the big 3 PBMs, a second interim report finds that PBMs heightened prices for important specialty generic drugs to increase their profit margins.
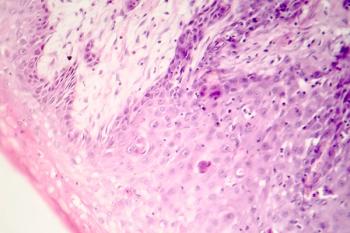
New data shows that, in high-risk patients with advanced cutaneous squamous cell carcinoma, cemiplimab reduces the risk of disease recurrence and death.

Wildfire smoke carries toxic compounds that can trigger respiratory inflammation and cause cellular damage.
Brentuximab vedotin is approved for treatment of adult and pediatric with classical Hodgkin lymphoma in combination with chemotherapy.
According to the investigators, BBO-8520 represents a first-in-class approach to treating patients with previously treated KRASG12C-mutated metastatic non-small cell lung cancer (NSCLC).
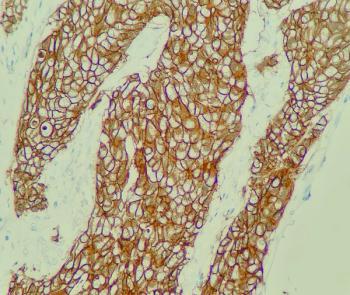
A new indication for the antibody-drug conjugate expands the future clinical potential of the treatment in patients with the rare breast cancer.
Datopotamab deruxtecan was also granted breakthrough therapy designation in December 2024.

The new Medicare cap limits out-of-pocket drug costs to $2000 annually.
The designation was supported by data from the phase 2, open-label, randomized, multi-center ARTEMIS-002 clinical trial, which assessed the safety and efficacy of GSK’227.
Pharmacists play a vital role in cancer prevention and early detection by educating patients on risk factors, counseling on screening guidelines, and promoting adherence to evidence-based recommendations for common cancers, such as breast, cervical, colorectal, lung, prostate, and skin cancer.

As of January 10, there are 5 major fires across a combined 36,560 acres—Palisades, Eaton, Kenneth, Hurst, and Lidia—and the 2 largest fires are a combined 11% contained.