The quadruple treatment was active and safe as initial therapy for older patients with transplant-ineligible multiple myeloma.

The quadruple treatment was active and safe as initial therapy for older patients with transplant-ineligible multiple myeloma.
γδ T cells are emerging as a transformative immunotherapy approach in oncology, offering unique mechanisms for targeting hematologic and solid tumors, with clinical trials demonstrating promising survival outcomes and durable immune responses.
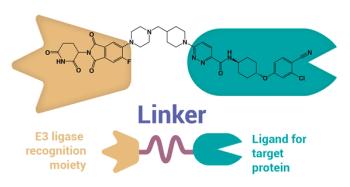
Proteolysis-targeting chimeras hold enormous potential, but also present significant challenges to drug developers.
Edward Kim, MD, MBA, discusses critical points of patient leakage in precision medicine, emphasizing the role of multidisciplinary teams in streamlining care, managing medications, and improving patient outcomes.
The Trop-2-tagreting antibody drug conjugate facilitated improved intracranial penetration with favorable tolerability.
Edward Kim, MD, MBA, highlights the challenges of patient disengagement, data fragmentation, and provider education in precision medicine, emphasizing the need for personalized approaches to enhance patient outcomes, particularly in oncology.
Encorafenib in combination with cetuximab and mFOLFOX6 met its dual primary end point of overall response rate of 61%.

The study results build on the “obesity paradox,” which suggests that patients with cancer and obesity have better outcomes.
Nivolumab plus ipilimumab demonstrated a 38% reduction in the risk of disease progression or death.
In the study, 50% of the deaths amongst patients were linked to central nervous system-related causes.
Overall, there is no long-term increased risk of developing thyroid cancer after initiating treatment with a glucagon-like peptide-1 (GLP-1) receptor agonist, although there is a short-term increase.

World Cancer Day, observed annually on February 4th, is a global initiative aimed at raising awareness, reducing stigma, and promoting equitable access to cancer care, with pharmacy professionals playing a vital role in prevention, treatment, and support for patients and communities.

Topical, targeted drug delivery increases drug concentrations at the desired site while also reducing toxicity and unwanted adverse effects.

Sara Rogers, PharmD, discusses the value of the Precision Medicine World Conference 2025 for pharmacy professionals, highlighting key sessions and opportunities aimed to advance precision medicine and pharmacogenomic (PGx) practices.
Naga Vara Kishore Pillarsetty, PhD, discusses advancements in radiopharmaceuticals for cancer therapy, noting common supply chain challenges, radiation safety measures, and patient misconceptions with the use of these drugs.

Judith Alberto, MHA, RPh, BCOP, discusses the COA 2025 Community Oncology Conference, emphasizing its focus on transparency in oncology and the role of data in supporting pharmacists' contributions to patient care.
The diagnostic identifies patients who may be eligible for treatment with fam-trastuzumab-deruxtecan-nxki (Enhertu; Daiichi-Sankyo, AstraZeneca).

Expert weighs in on data from the AQUILA trial and Johnson & Johnson's approval request for daratumumab for smoldering multiple myeloma.
Continuous dosing of ribociclib was associated with better tolerability and outcomes.

Myelofibrosis patients with CALR mutations have lower responses to symptoms and higher rates of anemia after 6 months of therapy with ruxolitinib.
Cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors have transformed the treatment of hormone receptor-positive/HER2-negative breast cancer across metastatic and early-stage settings, with ongoing research exploring their potential in additional breast cancer subtypes, combination therapies, and aggressive disease scenarios.
Digoxen is the active ingredient found in the foxglove plant and is commonly used to treat heart failure.

Lu-edotreotide (ITM-11; ITM) met its primary endpoint of prolonging progression-free survival (PFS).
Children and adults with cancer have differing immune system responses, mutational burdens, and tissue microenvironments.

Melody Chang, RPh, MBA, BCOP, explains how American Oncology Network leveraged its experience with the Oncology Care Model to address challenges when adopting the Enhanced Oncology Model.
The overall survival data were immature at the primary analysis of the study.

Diagnosing anemia, a serious and common complication of patients with myelofibrosis, could be made easier with the use of red cell distribution width assessment.

The supplemental new drug application is based on objective response rate and duration of response results from a phase 2 study that assessed the efficacy and safety of belzutifan.
Leukemia biology may predict patterns of blinatumomab failure after initial response.
As lung cancer cases rise, there is a significant need for therapeutic options that meet the challenges and needs of individual patients.