
A palliative care pharmacist highlights a spectrum of clinical opportunities for patients, caregivers, and clinicians when rounding at the bedside.

A palliative care pharmacist highlights a spectrum of clinical opportunities for patients, caregivers, and clinicians when rounding at the bedside.

Monica Sawiris, PharmD, said there were noticeable differences in toxicities between each regimen.

Erin Lexner, PharmD, BCPS, discussed her team’s experience transitioning to outpatient BEAM from inpatient CBV.

The high acceptance rates of these interventions demonstrate the key role in optimizing patient care that clinical oncology pharmacists with access to medical records play.
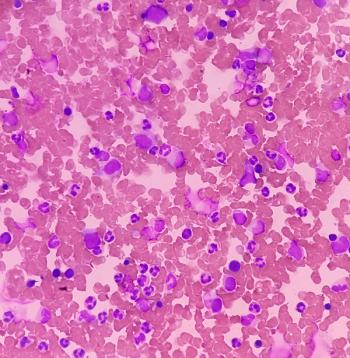
Current treatment strategies are aimed at reducing hematocrit and symptom burden through phlebotomy, aspirin, and cytoreductive agents, while emerging therapies offer promise for better disease control and quality of life.
Imlunestrant is an investigational, brain-penetrating, oral selective degrader of estrogen receptor.

Pharmacists share key insights about the use of ADCs for treatment of breast cancer.
There are no clearly defined, widely accepted clinical pharmacist metrics.

Christine Barrett, PharmD, BCOP, shares insights, challenges, and advice for oncology pharmacists navigating neoadjuvant therapy.

Scott A. Soefje, PharmD, MBA, BCOP, explained how the expanding use of the FDA’s 505(b)(2) drug approval pathway is creating operational and reimbursement challenges for oncology infusion centers.

Jessica Davis, PharmD, BCOP highlights the role of pharmacy technicians in ambulatory oncology clinic settings.

Patients with unresectable or metastatic hepatocellular carcinoma (HCC) receiving this regimen had favorable median overall survival (23.7 months) compared with standard of care (20.6 months).

In business, growth for the sake of growth mirrors the ideology of the cancer cell.

This approval marks bevacizumab-nwgd as the sixth reference product to bevacizumab (Avastin).

Metrics should empower pharmacists rather than burden them.


Lauren Dietz, PharmD, highlighted the role of oncology pharmacists in guiding prophylaxis decisions to optimize transplant outcomes.

Julia Ziegengeist, PharmD, BCOP offers insights for pharmacists navigating adjuvant and neoadjuvant treatment of breast cancer.
Christine Barrett, PharmD, BCOP, addressed advances in the treatment of stage III melanoma that provide the potential to improve long-term outcomes by enhancing immune response, tailoring surgical approaches, and refining patient selection strategies.
Mark Zangardi, PharmD, MS, BCOP, and Amber Cipriani, PharmD, BCOP, discuss the growing role of circulating tumor DNA (ctDNA) testing in oncology, highlighting its applications in early cancer detection, molecular residual disease assessment, and treatment monitoring.
The approval is for those with metastatic disease or in whom surgical resection is likely to result in severe morbidity, have no satisfactory alternative treatments, or have progressed following treatment.

Alexandra Wolff, PharmD, BCOP, FHOPA, was awarded a fellow of HOPA at the 2025 annual meeting.

Mya Tran, PharmD, BCOP, offers insights for navigating the complex treatment landscape for non-small cell lung cancer.

Michael Arnold, PharmD, discussed the role of oncology pharmacists in enhancing biomarker testing for patients with metastatic colorectal cancer.

Matthew Lei, PharmD, BCOP, highlights the value of community, collaboration, and innovation at the HOPA Annual Conference.
Sophie Jabban discusses research presented at the SGO Annual Meeting highlighting significant disparities in MRI access for patients with cervical cancer receiving chemoradiation, with delays linked to both race and insurance status, underscoring systemic barriers that may impact timely treatment.

The approval was based on efficacy and safety data from a randomized, 3-arm, open-label phase 3 CHECKMATE-8HW trial.

Enriqueta Felip, MD, PhD, discussed the benefits of subcutaneous pembrolizumab for patients with metastatic non–small cell lung cancer.
Fatty acid metabolism plays a critical role in treatment responses.
Sophie Jabban discusses findings from 2 studies that highlight how geographic location, race, ethnicity, and social determinants of health contribute to delays in initiating chemoradiation for patients with cervical cancer.