
Sara Roszak discusses her role as president of the NACDS Foundation.

Luke Halpern is an assistant editor with Pharmacy Times. Luke wrote for Pharmacy Times in the summer of 2023, and assumed a full-time role in June 2024. His work has been featured in Pharmacy Times and the American Journal of Managed Care. He graduated from the University of Massachusetts, Amherst in May 2024.

Sara Roszak discusses her role as president of the NACDS Foundation.

Scott Biggs and Doug Long explain current trends in the pharmaceutical industry and the importance of ensuring pharmacists are informed.
Jon Easter talks about community pharmacy's role in propelling diabetes innovation and optimizing patient care.

Rina Shah discusses the importance of community pharmacy and how pharmacists can optimize care for patients.
A federal and state policy expert discusses the announcement from the Centers for Medicare & Medicaid Services on the agreement to lower prices for 10 selected drugs due to the Inflation Reduction Act.
Building off the positive results of ELM-1, the ELM-2 trial found intravenously administered odronextamab was safe and effective in patients with relapsed or refractory follicular lymphoma.
The data builds off prior studies supporting bendamustine-rituximab as a first-line treatment for patients with non-Hodgkin lymphoma.
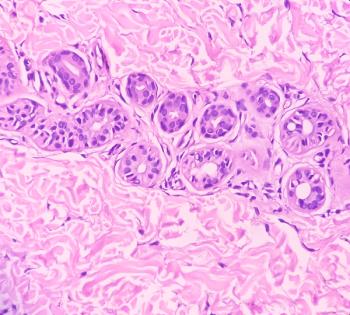
Nemolizumab is the first approved monoclonal antibody specifically inhibiting the signaling of IL-31, which drives disease mechanisms in prurigo nodularis.
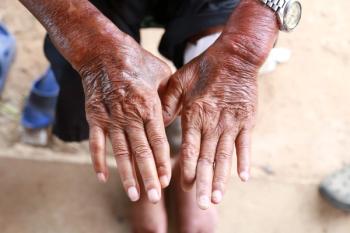
The systematic review summarized all available evidence on the subject, finding that intravenous immunoglobulin could have a role in treatment.
A variety of signs and symptoms of lymphoma were reported, including fever, stomach discomfort, and weight loss.

The FDA also granted rare pediatric disease designation for the drug.
Intravenous Immunoglobulin as a therapy for patients with pyoderma gangrenosum could open new treatment avenues for the rare and challenging condition.
The injection adds an additional option to address emergency known or suspected opioid overdoses.
Replacing carmustine with cisplatin in BEAM ((carmustine, etoposide, cytarabine, and melphalan) conditioning could be more cost-effective for patients.

Two different brands of intravenous immunoglobulin have been investigated, finding Brand P led to significantly longer durations of fever and hospitalization when compared to brand T.

Chris Peshek of Discount Drug Mart discusses the importance of community pharmacies and the process of building relationships with patients, and how a profession in the field can be rewarding for those seeking to pursue it.
The trial is evaluating the success of BNT111 and cemiplimab in treating unresectable stage III or IV melanoma whose disease had progressed following anti-PD-(L)1-containing treatment.

In the first half of 2024, the FDA approved 23 novel drugs for conditions including alopecia areata, Alzheimer disease, small cell lung cancer, bladder cancer, and more.
Investigators determine the efficacy and safety of the combination in a 2-phase trial.
In an analysis of 16 immunocompromised patients with COVID-19, intravenous immunoglobulin was effective and associated with clinical cure.
Afami-cel was approved in conjunction with MAGE-A4 IHC 1F9 pharmDx, a diagnostic tool that can identify patients eligible to receive the treatment for synovial sarcoma.
The RUBY trial will continue and analyze the overall population survival after treatment with the drug combination.

The presence of glutamic acid decarboxylase antibodies in intravenous immunoglobulin can lead to a misdiagnosis of type 1 diabetes mellitus if a false-positive result is garnered.
The designation is based off positive results from the RAMP 205 trial presented at the ASCO Annual Meeting.

Immunization rates have not returned to pre-pandemic levels, leaving millions of children and infants at risk for measles or diphtheria, tetanus, and pertussis.
A lack of widespread hepatitis B screenings compound with the issue of inaccurate test results, which can complicate treatment with intravenous immunoglobulin.
The new approach could optimize precision medicine and lead to better outcomes across a variety of disease states.

The FDA’s designation builds off positive results in a phase 1b/2a clinical trial, showing that the vaccine can effectively target the Tau protein.
Adherence and efficacy were both higher in patients with HIV who took twice-yearly lenacapavir compared to those on oral preexposure prophylaxis medication.
Emerging treatments, collaboration between health care providers, and new policy proposals have led to a positive outlook for the future of nonalcoholic steatohepatitis (NASH) and its treatment.