Brentuximab vedotin is approved for treatment of adult and pediatric with classical Hodgkin lymphoma in combination with chemotherapy.

Brentuximab vedotin is approved for treatment of adult and pediatric with classical Hodgkin lymphoma in combination with chemotherapy.
Datopotamab deruxtecan was also granted breakthrough therapy designation in December 2024.

The new Medicare cap limits out-of-pocket drug costs to $2000 annually.
Inclusion of AI improved detection and reduced instances of false-positive test results.

Limited access to healthy food in redlined communities contributes to higher rates of chronic disease and reduced life expectancy.

The late former president played a critical role in advancing cancer care, as well as efforts to eradicate preventable diseases.

Duchenne muscular dystrophy is an incurable neuromuscular disorder that can lead to cardiomyopathy, resulting in heart failure.

Palbociclib demonstrates safety and efficacy in combination with anti-HER2 and endocrine therapies.

This association was also observed in siblings of children with autism spectrum disorder.

The advisory emphasizes the need for a comprehensive approach to reducing alcohol-related cancer by combining prevention, education, and treatment to improve public health outcomes.
Sunvozertinib was originally approved in China, making it the world’s first and only oral treatment for these patients.

A pooled analysis showed regular consumption of coffee and tea was associated with decreased risk of certain cancers.
The patient was hospitalized after exposure to an infected backyard flock.
Two cases of hospitalizations show mutations that may allow H5N1 to more easily target human cells.
Calcipotriol in combination with 5-FU triggers Th2 immunity to prevent skin cancer.
The analysis shows patients achieved deep and durable responses despite being ineligible for the CARTITUDE-1 trial.
Patients with hormone receptor–positive and human epidermal growth factor receptor 2 breast cancer are particularly at risk.
Patients with relapsed, refractory disease achieved deep, durable responses with equecabtagene autoleucel.
The model, Deep-IO, shows improved specificity and sensitivity compared with existing predictive biomarkers.
The approval offers patients with solid tumors an alternative to intravenous PD-1 inhibitor administration.
Muvalaplin is an oral, small molecule inhibitor that can reduce lipoprotein(a).

High levels of the Oscillibacter genus of bacteria were associated with reduced cholesterol.
Gecacitinib is a novel inhibitor targeting both JAK and ACVR1.

Pharmacy Times attended the 2024 San Antonio Breast Cancer Symposium from December 10 to December 13.

Concizumab-mtci is a subcutaneous, monoclonal antibody designed to achieve hemostasis in all hemophilia types.
The nasal spray label resembles that of the FDA-approved epinephrine injection, leading to potential dosing errors.
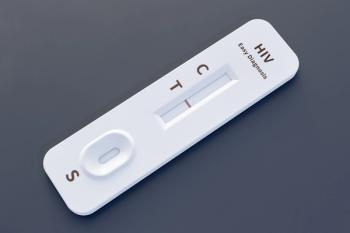
OraQuick was the first at-home HIV test kit to be approved by the FDA in 2012.
The decision is based off favorable results from the BREAKWATER trial.
Compounding pharmacies have a 60- to 90-day grace period to complete production as it continues to monitor supply and demand.

Duloxetine is a commonly prescribed medication used to treat anxiety, depression, and other mental health disorders.