
The analysis found that patients’ quality of life did not change significantly over time during treatment with immune checkpoint inhibitors.

The analysis found that patients’ quality of life did not change significantly over time during treatment with immune checkpoint inhibitors.

Dissimilarities include acceptance by third-party payers, applications, dispensation, prices, and use.

Tussin DM helps loosen phlegm and thin bronchial secretions to help drain bronchial tubes.
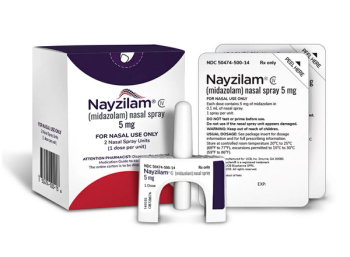
Midazolam is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity that are distinct from a patient’s usual seizure pattern in patients with epilepsy.
Chemotherapy for colorectal cancer include 5-fluorouracil (5-FU), capecitabine (Xeloda), irinotecan (Camptosar), oxaliplatin (Eloxatin), and trifluridine and tipiracil (Lonsurf).

Precision dosing technology uses multiple patient-specific factors to individualize medication doses, helping clinicians reduce medication errors that lead to adverse events.

Those aged 18 to 34 years self-treat with cannabidiol or cannabis at 2 times the rate of people aged 45 years or older.
The phase 2 Elara trial for the follicular lymphoma treatment met its primary endpoints, Novartis says.

Sacituzumab govitecan-hziy (Trodelvy) is indicated for the treatment of patients with triple-negative breast cancer who have received 2 prior therapies at minimum for metastatic disease.

Ten quiz questions to assess your knowledge on common symptoms and treatments for asthma.
J&J study results show that ustekinumab was beneficial in treating adults with the 2 types of IBD.
ARTISTRY-7 will analyze the anti-tumor activity and safety of the combination compared with chemotherapy for individuals with platinum-resistant ovarian cancer.
Investigators conclude that minimal residual disease cells, or those that survive the initial treatment, may carry some form of epigenetic and metabolic memory of the tumor.

Gyang and Hansen discussed any new or existing treatment options for patients, the role of the pharmacist changing, and what they wish others knew about MS.

Tinactin is an effective medication for athlete's foot, ringworm, and jock itch.


Apremilast is indicated for the treatment of psoriatic arthritis, plaque psoriasis, and oral ulcers associated with Behçet disease.
After skin cancer, breast cancer is the most common cancer diagnosed in women in the United States.
The multi-institutional clinic trial also concluded that there was no negative impact to quality of life for these patients.
The investigators said that the trial data showed potentially clinically meaningful improvements in both progression-free survival and overall survival in pre-specified subgroups of patients based on the baseline inflammatory biomarker, hs-CRP, as well as other biomarker-defined subgroups.

In this episode, Brooke Griffin, PharmD, founder of 21st Century PharmD, discussed how students have handled the pandemic, as well as how they can pursue leadership positions both during and after pharmacy school.

No timetable was set for the FDA to decide on an official EUA designation for the Pfizer-BioNTech vaccine in children 5 to 11 years of age, however a decision is expected within the next few days.
The research team analyzed links between pesticide exposure and the risk of kidney dysfunction in 41,847 people using data from the USA National Health and Nutrition Examination Survey.
An effective therapeutic treatment for acute or chronic pain needs to be convenient, easy to self-administer, and have rapid onset to provide fast relief.

The rate of distant metastasis at 10 years was 29% for individuals whose scores demonstrated more aggressive disease compared with 13% for those who showed lower risk.
The Keep Up the Rates campaign is aiming to encourage patients to catch up on routine vaccinations that might have been missed or delayed due to the COVID-19 pandemic.