
The FDA has approved golimumab for the treatment of patients 2 years of age and older with active polyarticular juvenile idiopathic arthritis.

The FDA has approved golimumab for the treatment of patients 2 years of age and older with active polyarticular juvenile idiopathic arthritis.

Inflammation caused by rheumatoid arthritis may increase the risk of type 2 diabetes.

The FDA has approved tofacitinib for the treatment of children and adolescents 2 years and older with active polyarticular course juvenile idiopathic arthritis.
A recent study found that patients on immunosuppressive therapy for common skin and rheumatic diseases, such as psoriasis and rheumatoid arthritis, are not at an increased risk for contracting COVID-19.
Recent studies have analyzed how individuals with autoimmune conditions prescribed specialty medications may be affected by the COVID-19 pandemic.

The Mediterranean diet has shown efficacy in preventing several noncommunicable diseases.
The new studies found that for some patients, the use of steroid medications to reduce inflammation slighly increased the likelihood of needing hospital care for COVID-19.
Extended glucocorticoid use can cause complications such as cardiovascular disorders, infections, and osteoporosis, according to a new study.
What characteristics of RA treatments possibly connect to COVID-19 treatment?
The American College of Rheumatology addresses the prior authorization requirement for some prescription medications, highlighting the significant burden this creates for patients and rheumatology professionals.

The ErbB inhibitors, gefitinib, erbstatin, and tryphostin AG825, inhibit a cell signaling process controlling the death-rate of neutrophils, immune cells, which cause damage to the lungs.

Officials with the FDA have approved Amgen’s infliximab-axxq (Avsola), a new biosimiliar to infliximab (Remicade).
It is currently uncertain to what extent interleukin inhibitors may increase the risk of serious infections and cancer in patients being treated for rheumatic diseases.

The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) agreed on a positive opinion for AbbVie’s upadacitinib (RINVOQ), according to the company.

Officials with the FDA have approved AbbVie’s upadacitinib (Rinvoq) 15 mg, once-daily oral Janus kinase (JAK) inhibitor, for the treatment of adults with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to methotrexate (MTX-IR).

Upadacitinib (Rinvoq, AbbVie) is indicated for adults with moderate-to-severe rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate.
Tofacitinib shows promising results in adult patients with moderately to severely active rheumatoid arthritis as a monotherapy with or without methotrexate.
Research investigates whether statins are safe and effective for patients with inflammatory conditions, such as rheumatoid arthritis.
A 10 mg twice daily dose of tofacitinib (Xeljanz, Xeljanz XR, Pfizer) used in patients with rheumatoid arthritis increased the risk of blood clots in the lungs and death in a safety clinical trial.

The higher dose is only approved for patients with ulcerative colitis, and not for RA.

The New Drug Application for upadacitinib is supported by data from the phase 3 SELECT trial program evaluating patients with moderate-to-severe rheumatoid arthritis.

Rituximab is approved by the FDA for the treatment of adults with rheumatoid arthritis, non-Hodgkin's lymphoma, chronic lymphocytic leukemia, pemphigus vulgaris, granulomatosis with polyangiitis and microscopic polyangiitis.
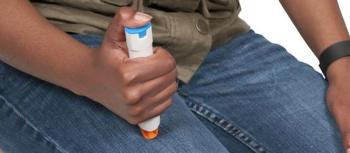
The single-dose, prefilled autoinjector for tocilizumab (Actemra) offers an additional option for patients with rheumatoid arthritis, giant cell arteritis, and 2 forms of juvenile arthritis.

New data presented at the 2018 ACR/ARHP Annual Meeting shows that the treatment regimens for many patients are not being changed to reach a “treat-to-target†goal for low disease activity.

A look at last week's top stories in the world of pharmacy.