Cox-2 inhibitors carry risk for arthritis patients with heart disease.

Cox-2 inhibitors carry risk for arthritis patients with heart disease.
Top news of the day from across the health care landscape.
Patients who live with rheumatoid arthritis do not die from swollen joints but face twice the risk of suffering a heart attack or stroke.

The 12-biomarker test is gaining acceptance among rheumatologists and was recently added to a clinical guideline.

New therapeutic targets provide hope for targeted treatments for rheumatoid diseases.

JAK inhibitors may be more effective than older biologic drugs in certain patients.
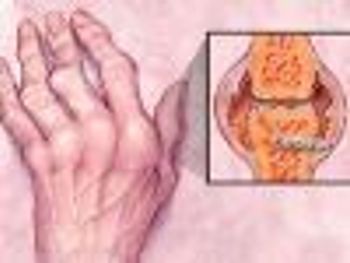
A recent review outlines the best treatment option to prevent bone loss associated with rheumatoid arthritis.

Patients taking baricitinib saw improvements in rheumatoid arthritis symptoms as soon as a week into treatment.
Orencia is the only CTLA-4lg approved in the United States, European Union, and Japan for the treatment of rheumatoid arthritis.

Orencia is a fusion protein and the only CTLA-4lg approved in the United States for the treatment of rheumatoid arthritis.

The FDA discovered deficiencies in a Sanofi manufacturing facility for the rheumatoid arthritis drug sarilumab.

The FDA discovered deficiencies in a Sanofi manufacturing facility for the rheumatoid arthritis drug sarilumab.

Top news of the day from across the health care landscape.

Inflectra is the first biosimilar monoclonal antibody approved by the FDA.

Top news of the day from across the health care landscape.
Adalimumab also found to be effective in treating non-infectious uveitis.
Baricitinib monotherapy or in combination with methotrexate shows superiority treating rheumatoid arthritis.

Investigational drug used alone or in combination demonstrated superiority to methotrexate in patients with active rheumatoid arthritis.

Adherence programs needed to boost Xeljanz utilization for rheumatoid arthritis.

Amgen’s biosimilar Amjevita approved for variety of anti-inflammatory diseases, however, ongoing litigation may prevent it from hitting the market.

Refunds will be offered to plan sponsors if a patient discontinues a preferred anti-inflammatory drug within 90 days.

Erelzi, an etanercept biosimilar, was approved for rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and polyarticular juvenile idiopathic arthritis.

Sandoz has gained FDA-approval for Erelzi, an etanercept biosimilar.

Patients taking medication that inhibits IL-1beta are 300 times more likely to develop a flesh-eating infection.

Top news of the day from across the health care landscape.