Lymphoma
Latest News
Latest Videos

CME Content
More News
In addition, patients who were treated with liso-cel therapy had a higher average life expectancy and QALYs, compared to those who were treated with standard care.
The authors note that CAR-T cell therapy could change the treatment standard for patients with DLBCL, who previously had few options if standard chemotherapy treatment was ineffective.

Currently, there is no established standard of care treatment approach of HGBL, with frontline interventions consisting of chemo-immunotherapies.

The update followed results from the phase 1/2 EPCORE NHL-1 clinical trial evaluating the safety and preliminary efficacy of the drug, including in individuals with relapsed or refractory follicular lymphoma.
Leung discusses that various cancer types and drugs can cause thrombotic microangiopathy and impact kidney health and the renal complications connected to hematologic malignancies.
Axicabtagene Ciloleucel Demonstrates High Response Rate in Relapsed/Refractory Large B-cell Lymphoma
Axicabtagene ciloleucel achieved a complete metabolic response of 71% at 3 months versus 12% expected with standard of care among transplant ineligible patients with relapsed/refractory large B-cell lymphoma.

Improved classification of different lymphoma types can improve the potential of targeted therapies and help predict patient responses.
The use of base editing to generate universal, off-the-shelf CAR T cells is a promising approach for relapsed leukemia, with potential implications for the future of gene therapy.
Bispecific antibodies have offered a new treatment approach in relapsed/refractory multiple myeloma, with promising results in preclinical studies for multiple cancers and hematological malignancies.
A low dose of the combination treatment inhibited AML cell cycle and increased apoptosis.
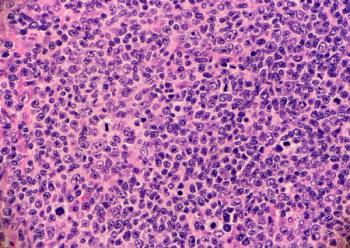
Correctly diagnosing specific follicular lymphomas and marginal zone lymphomas requires knowledge of the clinical context, including the site of involvement, age, and clinical presentation.
Patients with lymphoma can be immunocompromised due to their disease, anti-cancer therapy, and concomitant immunosuppressive treatments, which make them more vulnerable to developing a COVID-19 infection.

Investigators found that artificial intelligence chatbots did not consistently provide recommendations for cancer treatment that correspond with NCCN guidelines.

Although additional studies are needed, findings indicate that mtDNAfb may be a useful biomarker for future risk of non-Hodgkin lymphoma.

Lisaftoclax Granted FDA Clearance for Phase 3 Trial in Patients With Chronic Lymphocytic Leukemia, Small Lymphocytic Lymphoma
Lisaftoclax is a novel, oral, small-molecule, BCL-2 selective inhibitor being evaluated for treatment of relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma.

Pembrolizumab Produces a Sustained Response in Patients With Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma
At a median follow-up of 48.7 months, pembrolizumab monotherapy produced an investigator-assessed objective response rate of 41.5% and a 20.8% complete response rate in patients with relapsed/refractory primary mediastinal large B-cell lymphoma.
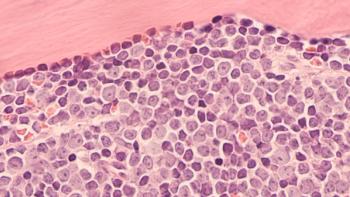
microRNAs have shown potential to be used as biomarkers for diagnosing and treating different types of cancers, though their application in doing so had yet to be determined.
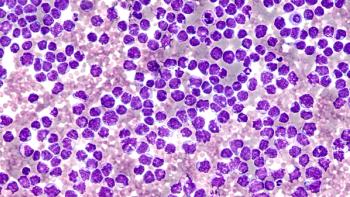
Investigators identified a specific subgroup of patients with mantle cell lymphoma who were at higher risk by defining a combination of MIPI, Ki-67, and p53/TP53 alternations.
Due to the assessment of computerized tomography with criteria from 1999, the role of radiotherapy in patients with diffuse large B-cell lymphoma with bulky or extranodal disease remains undefined.

The phase 3 S1826 trial is the largest Hodgkin lymphoma study in NCTN history.
Zanubrutinib combined with obinutuzumab was previously granted both Fast Track and Orphan drug designations for patients with relapsed or refractory follicular lymphoma.

Irreversible Bruton tyrosine kinase inhibitor produces positive results in patients with relapsed/refractory marginal zone lymphoma.

Pirtobrutinib is a non–covalent (reversible) BTK inhibitor, demonstrating a 73.3% overall response rate with a median follow-up of 19.4 months among patients with chronic lymphocytic leukemia or small lymphocytic lymphoma.

The recommended addition is based on a uniform consensus that the therapy is an appropriate treatment for this difficult-to-treat disease.

The approval was based on data from 2 studies of blinatumomab in adults and pediatric patients with CD19-positive B-cell precursor acute lymphoblastic leukemia.