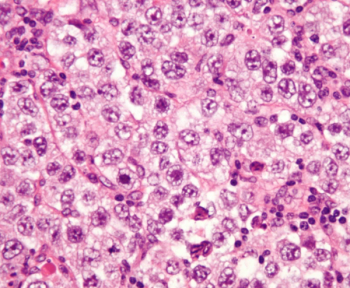
Lisocabtagene maraleucel is a CD19-directed CAR T-cell therapy with a 4-1BB costimulatory domain, which enhances the expansion and persistence of the CAR T cells.

Lisocabtagene maraleucel is a CD19-directed CAR T-cell therapy with a 4-1BB costimulatory domain, which enhances the expansion and persistence of the CAR T cells.

The FDA has approved pirtobrutinib (Jaypirca) for the treatment of adult patients with relapsed or refractory mantle cell lymphoma following at least 2 lines of systemic therapy, including a Bruton tyrosine kinase inhibitor.

Chronochemotherapy aims to time drug delivery when the body is the least vulnerable to harmful effects, while the cancer cells are the most vulnerable.
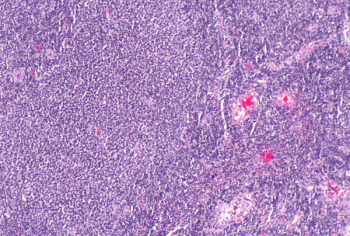
At leukapheresis, a low frequency of differentiated CD3+CD27-CD28--T cells can indicate favorable response to CAR T-cell therapy.

Bruton’s tyrosine kinase inhibitor zanubrutinib (Brukinsa; BeiGene USA, Inc) approved for the treatment of chronic lymphocytic leukemia or small lymphocytic lymphoma.

If approved, glofitamab will treat the most aggressive type of non-Hodgkin lymphoma.
Participants who survived Hodgkin lymphoma as children showed signs of being biologically older than their peers, with a heightened risk of cognitive problems.

The phase 2 GO29781 study showed that 80% of patients who had received at least 2 prior therapies achieved durable response rates with mosunetuzumab-axgb treatment.

Medical experts open a discussion on treatment management for patients with CP-CML and Ph+ ALL.
E7777 is an engineered interleukin-2-diphtheria toxin fusion protein that is a purified and more bioactive formulation of Ontak, which the agency previously approved.

Expert discusses the updated data for a trial cohort after a median follow-up of 27 months.
Based on real-world outcomes, there is an unmet need for an effective therapy to be used among patients aged 75 years or older with relapsed/refractory diffuse large B-cell lymphoma.

Although patients with Richter syndrome often experience poor outcomes, there are many new treatment strategies that have demonstrated a durable response.
Expert discusses clinical outcomes of the phase 3 ZUMA-7 trial assessing axicabtagene ciloleucel versus standard-of-care in second-line large B-cell lymphoma by metabolic tumor volume.

Expert discusses the safety and efficacy of polatuzumab vedotin combined with rituximab, ifosfamide, carboplatin, and etoposide (R-ICE) as second-line treatment in a multicenter phase 2 study.

In recent decades, clinical trials have grown increasingly restrictive and exclusive, which has impacted the inclusivity and diversity of selected trial participants.

Study finds textured breast implants do not increase the risk of anaplastic large-cell lymphoma, which has grown in incidence since 2008.

The allogeneic CAR-T cell therapy is in development for relapsed or refractory large B cell lymphoma and relapsed or refractory B cell non-Hodgkin lymphoma.

Olutasidenib (Rezlidhia) is indicated for adult patients with relapsed or refractory acute myeloid leukemia with a susceptible IDH1 mutation detected by an FDA-approved test.

An experimental drug could help scientists understand the development of mantle cell lymphoma and potentially increase overall survival.
Zanubrutinib expressed better cardiac safety measures, higher progression-free survival, and lower discontinuation rates in the ALPINE trial compared with compared ibrutinib.

Agency’s action date is May 21, 2023, for the first subcutaneous bispecific antibody approved for the treatment of large B-cell lymphoma.

FDA approves brentuximab vedotin for the treatment of patients aged 2 years and older with previously untreated, high-risk classical Hodgkin lymphoma, in combination with doxorubicin, vincristine, etoposide, prednisone, and cyclophosphamide.

Study shows combining brentuximab vedotin with the standard chemotherapy regimen reduced the risk of relapse in children with high-risk Hodgkin lymphoma by 10%.

In the inpatient acute care setting, oncology pharmacists maximize return on investments of oncology products, produce optimal outcomes, and ensure that quality of care is consistent and safe.