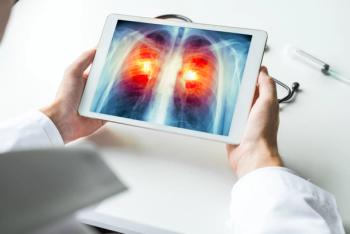
Sotorasib (Lumakras) plus carboplatin and pemetrexed shows promise in the first line treatment of advanced non–small cell lung cancer.
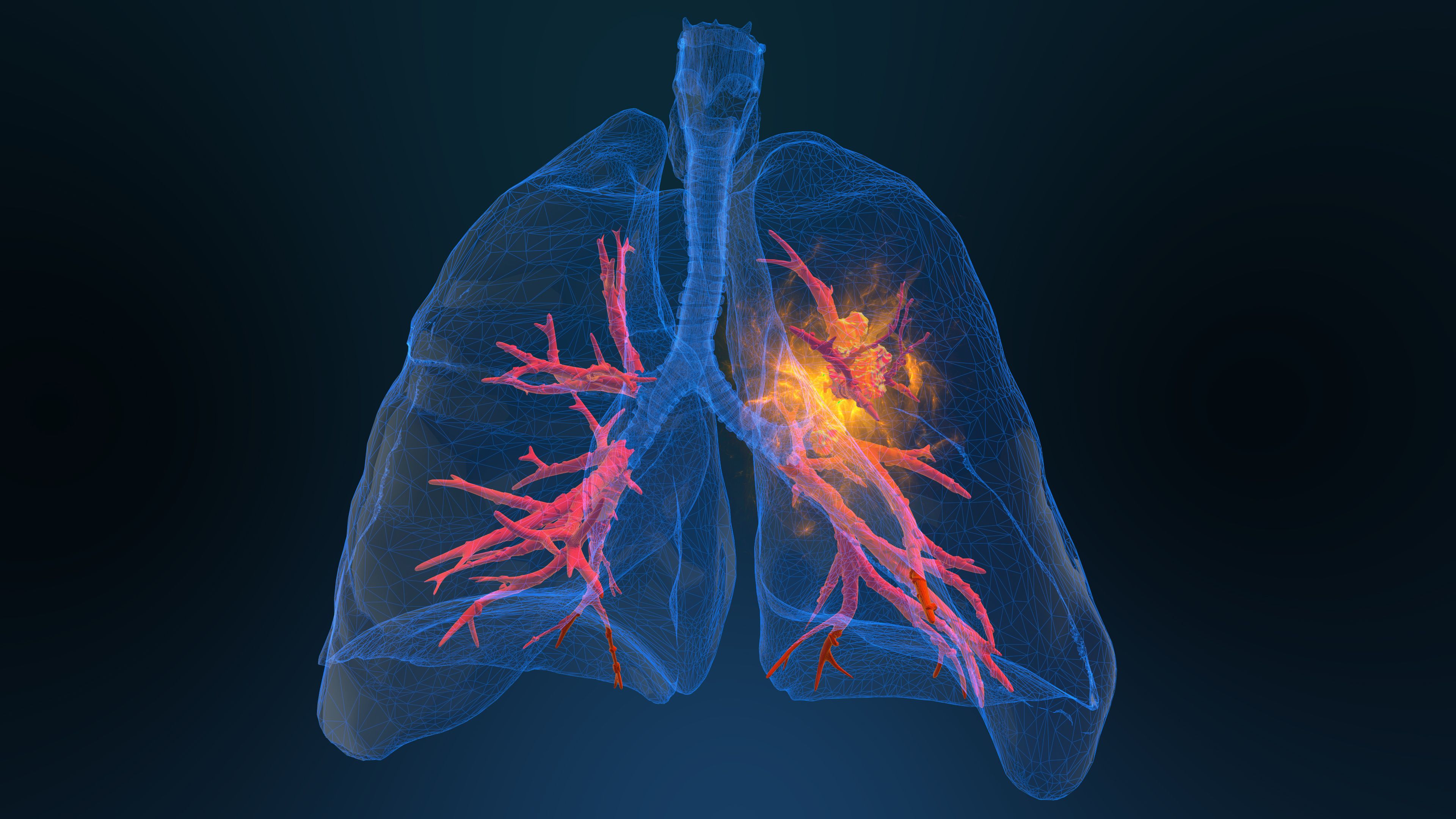

Sotorasib (Lumakras) plus carboplatin and pemetrexed shows promise in the first line treatment of advanced non–small cell lung cancer.

Compared to chemotherapy alone, the combined nivolumab and ipilimumab treatment more than tripled the overall survival rate at 6 years in patients with PD-L1 expression <1%.
The study met its primary endpoint of progression-free survival, and overall survival is continuing to be evaluated.

Patients who received amivantamab plus chemotherapy or amivantamab without chemotherapy presented better outcomes compared to chemotherapy alone.
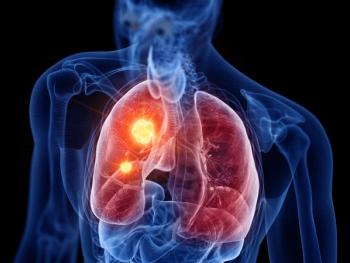
If approved, alectinib would be the only anaplastic lymphoma kinase inhibitor to show reductions in risk of recurrence or death for those with early-stage lung cancer.

Patients with cancer and survivors of cancer that had engaged in administrative tasks to effectively estimate costs or pay for care was associated with an 18% increase in cost-related treatment delays or treatment nonadherence.
The supplemental biologics license application for amivantamab-vmjw (Rybrevant) is supported by data from the phase 3 PAPILLON trial, evaluating the efficacy and safety of the drug in combination with chemotherapy for those with non–small cell lung cancer.

Investigators found that artificial intelligence chatbots did not consistently provide recommendations for cancer treatment that correspond with NCCN guidelines.

The digital cessation platform is the first FDA-cleared OTC device to help individuals quit smoking tobacco.
In the study, investigators incorporated data from electronic health records and social determinants of health into the model to determine the likelihood in delays when starting cancer therapy.
Based on results from a trial of repotrectinib in patients with ROS1-positive locally advanced or metastatic non-small cell lung cancer, the FDA accepted the New Drug Application and set a Prescription Drug User Fee Act goal date of November 27, 2023.

Though an association was found between depression and anxiety and lung cancers, there were no connections found to overall, breast, prostate, colorectal, and alcohol-related cancers.
Amivantamab-vmjw in combination with chemotherapy could assist patients diagnosed with non–small cell lung cancer when chemotherapy alone did not show benefit.
The 5-year survival rate for patients with exon 20 insertion EFGR mutation is 8% in the frontline setting, worse than the survival rate of the 2 most common types of EGFR mutations in patients with advanced or metastatic non-small cell lung cancer.

A panel at the Advanced Topics for Oncology Pharmacy Professionals Summit discusses strategies to address patients’ fears and distrust of the medical and scientific community.

A cancer vaccine has shown efficacy in dogs, with implications for benefit in humans.
Updated phase 3 trial data provide practice-changing findings.

Oncology pharmacy is best learned in real time with real oncologists and real patients.

Trial shows an improved survival rate at 4 years when treated with the STRIDE regimen in comparison to treatment with sorafenib in patients with unresectable hepatocellular carcinoma.
Despite recommendations for adults to receive lung cancer screenings, rates have remained low compared with other cancer screening programs.
More than half of the patients prescribed oral anticancer agents report persistent moderate-to-severe symptoms, despite receiving counseling on adverse effect management.

Race, ethnicity, years after diagnosis and mental and physical stress are among factors associated with unmet supportive care needs and a higher risk of hospitalization among patients with cancer.
Approximately every 2.2 minutes, a new diagnosis of lung cancer is registered.

More than 4 times as many people who used the app quit smoking compared to a traditional support service.
Panelists discuss the potential impact of the study data presented at the ASCO 2023 Annual Meeting, which led to significant response from the field.