AfterBurn gel offers relief for sunburn, scalds, and other types of burns.

AfterBurn gel offers relief for sunburn, scalds, and other types of burns.
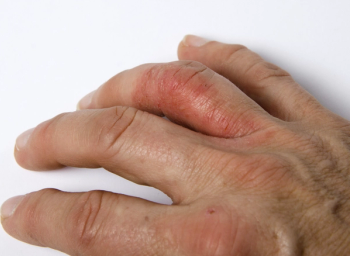
People living with psoriatic arthritis deserve to be informed about ways to approach disease management holistically and with a personalized approach.
Alocane provides fast pain relief and helps the healing process from minor burns.
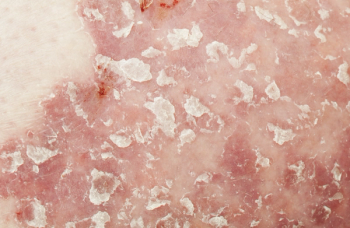
Tapinarof is a first-in-class topical steroid-free aryl hydrocarbon receptor antagonist intended to mitigate the symptoms of plaque psoriasis.

TriDerma Eczema Fast Healing helps sooth dry and irritated skin for itch relief.

Indications for adalimumab include ankylosing spondylitis, Crohn disease, chronic plaque psoriasis, juvenile idiopathic arthritis, moderate to severe rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.

Ruxolitinib (Opzelura; Incyte) cream 1.5% is the first and only treatment approved by the FDA for repigmentation in patients with vitiligo.

Tildrakizumab-asmn is a humanized IgG1/k monoclonal anti-IL-23 antibody indicated for the treatment of adults with moderate-to-severe plaque psoriasis.
Oritavancin (Orbactiv) is indicated for the treatment of adult patients with acute bacterial skin and skin structure infections caused or suspected to be caused by susceptible isolates of designated Gram-positive microorganisms.

Desitin is used to provide relief of skin irritation caused by a diaper rash.

Calmoseptine is used to treat irritation caused by skin rashes.
Tildrakizumab-asmn (Ilumya) is indicated for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy.

BurnAid burn dressing provides relief for all types of minor burns.

After Bite is indicated for relieving itch from bug bites and other mild irritations.
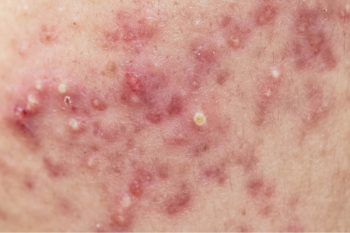
Isotretinoin exerts dose-dependent inhibition of sebaceous gland function, thereby reducing sebum secretion, which is associated with improvement in nodular acne.

Tecnu Rash Relief Spray treats skin irritation caused by sunburn, minor skin irritations, insect bites, and other minor skin issues.

The topical therapy demonstrated significant improvements in the study, based on IGA success and other endpoints, according to investigators.
Clinically meaningful results may be a huge step forward in the development of a new therapy for systemic lupus erythematosus.

Better Nail is an anti-fungal product for fungus under and around the nails.

Baricitinib (Olumiant; Eli Lilly and Company) is the first systemic treatment to be approved by the FDA for severe alopecia areata, which affects an estimated 300,000 patients in the United States.

Approval in this patient population makes dupilumab the first and only biologic medicine approved for the treatment of moderate-to-severe atopic dermatitis from infancy through adulthood.
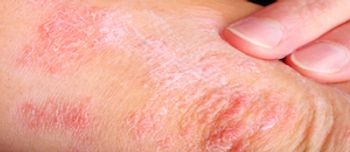
Further analyses showed guselkumab provided patients with sustained improvements in measures of health-related quality of life, fatigue, pain, and work productivity.

Dupilumab is under clinical development, and the efficacy and safety have not been fully evaluated by any regulatory authorities.

Study findings provide evidence that preventative antibiotics improve health outcomes for babies and mothers, investigator say.
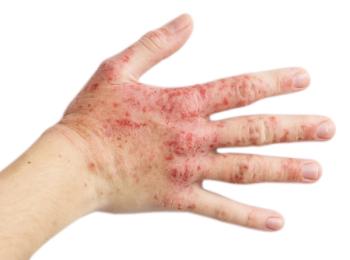
The FDA has approved abrocitinib tablets to treat adults with moderate-to-severe, refractory atopic dermatitis.