
Roflumilast is the first topical agent indicated to treat psoriasis in sensitive intertriginous areas of the skin.

Roflumilast is the first topical agent indicated to treat psoriasis in sensitive intertriginous areas of the skin.
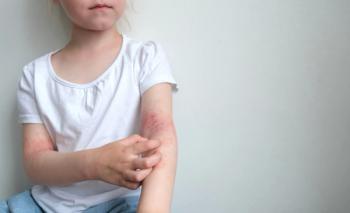
A supplemental New Drug Application will be submitted for roflumilast cream 0.05% for the treatment of mild to moderate atopic dermatitis in children 2 to 5 years of age.

August's Condition Watch focuses on skin health.

Although SB5 is an effective treatment for plaque psoriasis, it is recommended that patients consider the potential risks and adverse effects.

Results of phase 3 trials could lead to the approval of remibrutinib to provide more effective and controlled treatment of chronic spontaneous urticaria than H1-antihistamines.
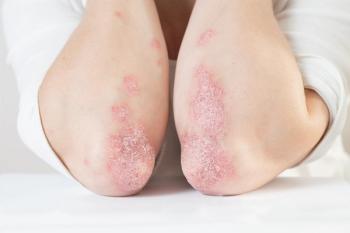
Patients on risankizumab also reported greater satisfaction with treatment for moderate plaque psoriasis.
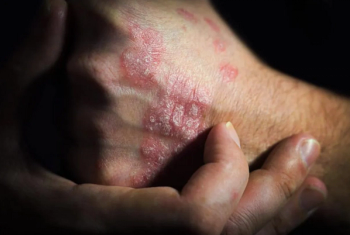
FDA approvals of these biosimilars started in 2016, but only 1 such product has successfully launched.
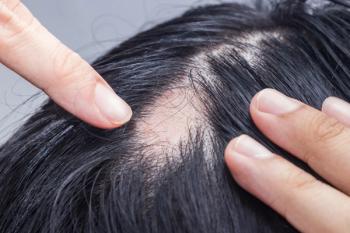
This approval makes ritlecitinib, in the 50 mg dosage, the first and only treatment approved by the FDA for adolescents aged 12 years and older with severe alopecia areata.
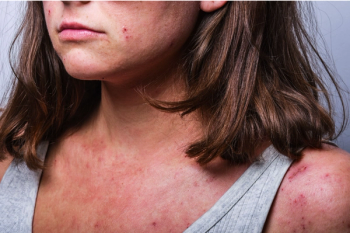
Acne, atopic dermatitis, rosacea, urticaria, and sunburn present differently and require specific management.

Previously, upadacitinib improved symptoms through 2 years in the SELECT-PsA 1 trial for those with psoriatic arthritis with prior inadequate response or intolerance to ≥1 non-biologic disease-modifying antirheumatic drug.

Pediatric drug development, which has a complex background and history, has fundamental catches based around how children are defined in the context of clinical studies.

This is the first clinical evidence to show that stimulating the programmed cell death protein 1 (PD1) inhibitory pathway is beneficial for rheumatologic disease.

Skinvive by Juvéderm is a first of its kind medication to create skin smoothness that lasts 6 months with optimal treatment.
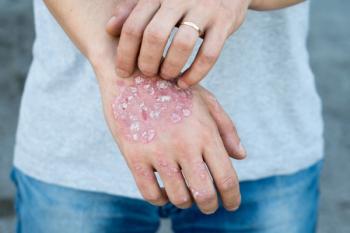
Due to the disease’s chronicity, the variability in treatment response and drug safety profiles, and the availability of multiple therapies, switching or discontinuing treatments is common in psoriasis.

Clearer skin on the face and hands lasted 1 year into treatment.

Pharmacists play key roles in navigating prior authorizations and other limits on utilization, as well as educating and listening to patients and caregivers.

Muneeb Shah, DO, dermatologist and partner at PC Dermatology PLLC, and Reid Maclellan, MD, founder of Cortina Health Inc also discuss the role technology and social media play in health care education.
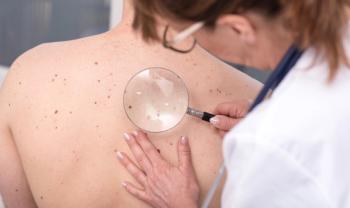
Muneeb Shah, DO, dermatologist and partner at PC Dermatology PLLC, and Reid Maclellan, MD, founder of Cortina Health Inc, tackle misinformation and myths surrounding sun screen, skin cancer, and acne.

Phase 2B clinical trial results demonstrate that patients experienced improvement in total body and facial repigmentation.
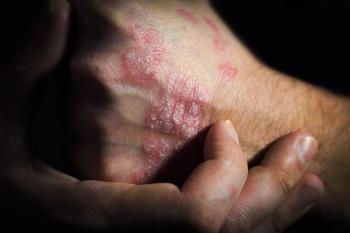
In an additional post-hoc analysis, guselkumab demonstrated improvements at week 48, according to Janssen Pharmaceutical.

More than 300,000 individuals in the United States have CSU that is inadequately controlled by antihistamines.
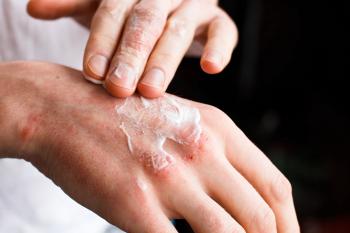
Results of 2 phase 3 clinical trials demonstrate better response rate beyond the primary endpoint analysis at week 16 to more than 55 at week 55.

Expanded indication for abrocitinib (Cibinqo; Pfizer Inc) includes patients 12 to <18 years of age with refractory, moderate-to-severe atopic dermatitis that is not adequately controlled with other systemic medications.
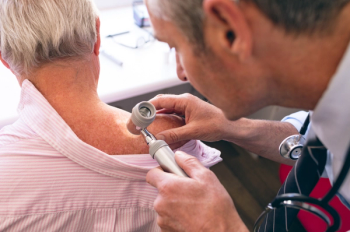
Several studies have noted a potential link between methotrexate and an increased risk of skin cancer.
Results from the phase 2 and long-term trials will be presented later this year, following further data collection.