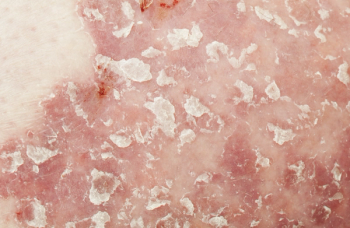
Tildrakizumab-asmn is a humanized IgG1/k monoclonal anti-IL-23 antibody indicated for the treatment of adults with moderate-to-severe plaque psoriasis.

Tildrakizumab-asmn is a humanized IgG1/k monoclonal anti-IL-23 antibody indicated for the treatment of adults with moderate-to-severe plaque psoriasis.

Imlunestrant is currently under investigation as monotherapy or in combination with other anticancer therapies for patients with estrogen receptor-positive, locally advanced or metastatic breast cancer and endometrial cancer.

Cabotegravir inhibits HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral DNA integration.

The FDA recently approved cabotegravir 200 mg/mL and rilpivirine 300 mg/mL as a once-monthly treatment for adults with HIV-1 who are virologically suppressed on a stable antiretroviral regimen.
Published: July 18th 2022 | Updated: