Dermatology
Latest News
Latest Videos

CME Content
More News
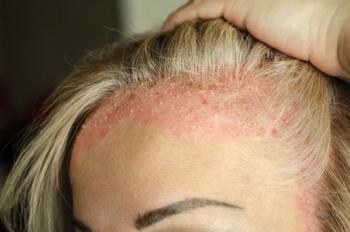
The FDA assigned a Prescription Drug User Fee Act action date of May 22, 2025.

If approved, delgocitinib cream would be the first US treatment indicated for moderate to severe chronic hand eczema.
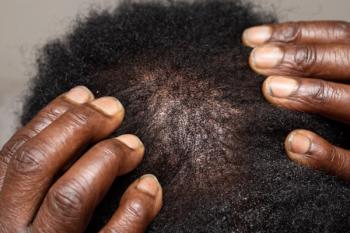
There is a need for continued research and awareness in the diagnosis and management of alopecia in diverse populations.

Lebrikizumab (Ebglyss; Eli and Lilly Company) is a monthly maintenance injection with proven efficacy in adults and children aged 12 to 18 years.
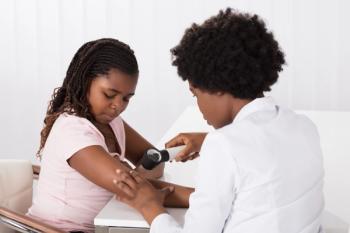
Not only do diverse clinical cohorts improve the strength of the data, but it improves public trust in clinicians and the medical system as a whole.

The company also submitted data from the ongoing phase 2 study, demonstrating the improvements of the investigational drug for symptoms of overall disease severity.
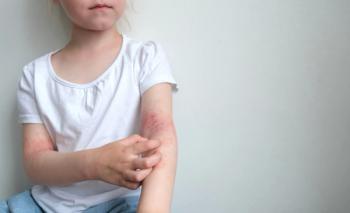
In July 2024, the FDA approved the supplemental new drug application for roflumilast cream 0.15% for patients 6 years and older.
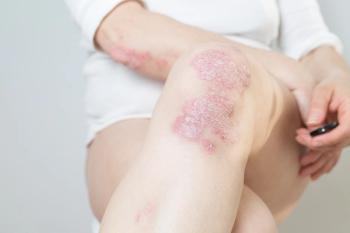
Pediatric patients weighing 20 kg to <50 kg should take 20 mg of apremilast twice daily and children weighing at least 50 kg should take 30 mg twice daily.


The study authors recommend that health care professionals are aware of the connections, notably in adolescent patients and those with moderate to severe psoriasis.
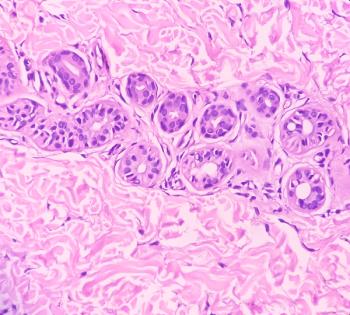
Nemolizumab is the first approved monoclonal antibody specifically inhibiting the signaling of IL-31, which drives disease mechanisms in prurigo nodularis.
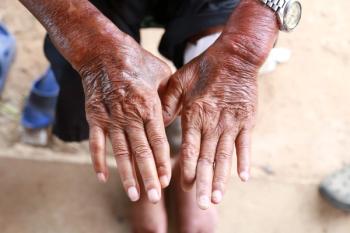
The systematic review summarized all available evidence on the subject, finding that intravenous immunoglobulin could have a role in treatment.
Intravenous Immunoglobulin as a therapy for patients with pyoderma gangrenosum could open new treatment avenues for the rare and challenging condition.
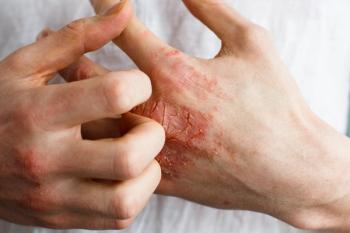
The Lancet published data from DELTA 1 and DELTA 2 trials that displayed treatment success among individuals treated with delgocitinib cream.
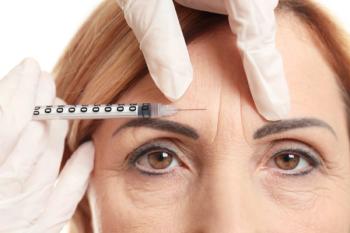
The decision expands the use of incobotulinumtoxinA beyond treatment of frown lines.

Individuals that followed the Mediterranean diet with EPA-DHA supplements displayed an increase in omega-3 levels which improved clinical appearance.


The importance of protecting the skin is often underestimated by patients

Roflumilast is approved as a once daily and steroid-free cream for rapid disease clearance and significant reduction in itch and for long-term disease control.

When initiated at a 15 mg dose and escalated to 30 mg, upadacitinib showed superiority compared with dupilumab, achieving Eczema Area and Severity Score (EASI) 90 at week 16.

Pharmacists are leveraging tools, campaigns, and free samples to educate patients about medication-induced photosensitivity and the importance of sun protection.

Hyperhidrosis affects an estimated 10 million individuals in the US and is characterized by abnormally increased sweating, beyond what is necessary to regulate body temperature.

The autoinjector will provide another option for adults in addition to the prefilled syringe that is currently available.
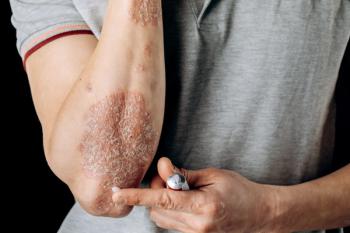
Reducing sodium intake is associated with decreased risk of atopic dermatitis (AD) and flares.

Parents and caregivers sometimes have concerns about their children receiving these injections, but researchers and experts assure that the FDA-approved biologic is safe and effective in young populations.