Chronic Lymphocytic Leukemia
Latest News
Latest Videos

CME Content
More News
Zanubrutinib expressed better cardiac safety measures, higher progression-free survival, and lower discontinuation rates in the ALPINE trial compared with compared ibrutinib.
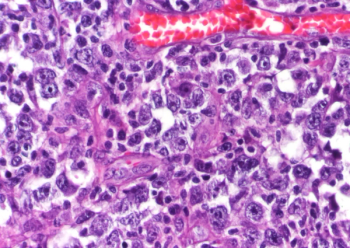
Investigators believe the findings can refine predictions, after independent evaluation, by identifying different modes of genetic alteration.

In the inpatient acute care setting, oncology pharmacists maximize return on investments of oncology products, produce optimal outcomes, and ensure that quality of care is consistent and safe.

Opportunities for further optimization of CML management remain.
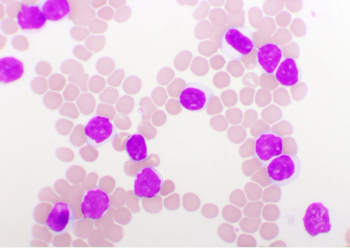
Zanubrutinib Shows Superior Progression-Free Survival vs Ibrutinib in Chronic Lymphocytic Leukemia
Zanubrutinib (Brukinsa) is a small molecule Bruton’s tyrosine kinase inhibitor under evaluation as a monotherapy and in combination with other treatments for various B-cell malignancies.
Omidubicel is a stem cell-based product that utilizes nicotinamide to inhibit differentiation and to increase the migration, bone marrow homing, and engraftment efficiency of hematopoietic progenitor cells.
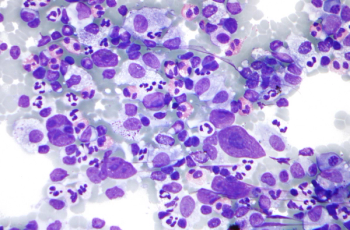
Currently available agents may reduce the need for stem cell transplantation in patients with acute lymphocytic leukemia.
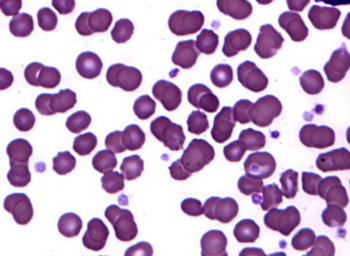
Relapses remain a challenge in treating patients with leukemia.
The functional and physical well-being and other scores from the survey are more favorable for those who were treated with the therapy, investigators say.

Expert discusses whether epigenetic programs regulate human T cell exhaustion and whether epigenetic-based strategies can be used to enhance T cell-based therapies.
This study highlights one benefit of the integrated health system specialty pharmacy model for patients who are prescribed acalabrutinib.

NVG-111 is a humanized, tandem single-chain variable fragment ROR1 x CD3 BiTE being evaluated in relapsed/refractory chronic lymphocytic leukemia and mantle cell lymphoma.

Both ibrutinib (Imbruvica) and venetoclax (Venclexta) carry an approved indication for use in chronic lymphocytic leukemia but do not often lead to complete remission, and therapy routinely continues indefinitely or until disease progression.

The nature of CLL being a long-term, incurable disease makes its treatment a challenge as it is more focused toward improving patient’s quality of life.

A patient with CLL relies on his pharmacy team for support.

Five-year progression-free survival in the CLL14 trial is approximately 63% in the targeted treatment arm, according to new data.
Overall, the investigators observed that the CR, undetectable minimal residual disease rates, progression free survival, and overall survival amomg the patients enrolled in the trial were favorable.
AstraZeneca trial results shows 90% of patients with chronic lymphocytic leukemia surviving for 5 years.
Lisa Nodzon, PhD, ARNP, AOCNP; Katie Tobon, PharmD, BCOP; and Javier Pinilla-Ibarz, MD, PhD, share insight on strategies for the monitoring and management of BTK inhibitor–associated toxicities in CLL and review the importance of a multidisciplinary approach to treatment.

Acalabrutinib (Calquence; AstraZeneca) provides efficacy while maintaining favorable tolerability for patients with chronic lymphocytic leukemia.

Lisa Nodzon, PhD, ARNP, AOCNP, and Javier Pinilla-Ibarz, MD, PhD, evaluate novel agents and combinations in the pipeline for the management of CLL.

Bhavesh Shah, RPh, BCOP, and Ryan Jacobs, MD, discuss emerging agents in the pipeline and the future treatment landscape for CLL.

Ryan Jacobs, MD, and Bhavesh Shah, RPh, BCOP, comment on the role of pharmacists in clinical practice for managing patients with CLL.

Katie Tobon, PharmD, BCOP, leads the discussion comparing dosing and drug interactions among the FDA-approved BTK inhibitors ibrutinib and acalabrutinib for the treatment of CLL.

Key opinion leaders in hematology-oncology share recommendations on switching from first-generation BTK inhibitors to next-generation BTK inhibitors for the management of patients with CLL.