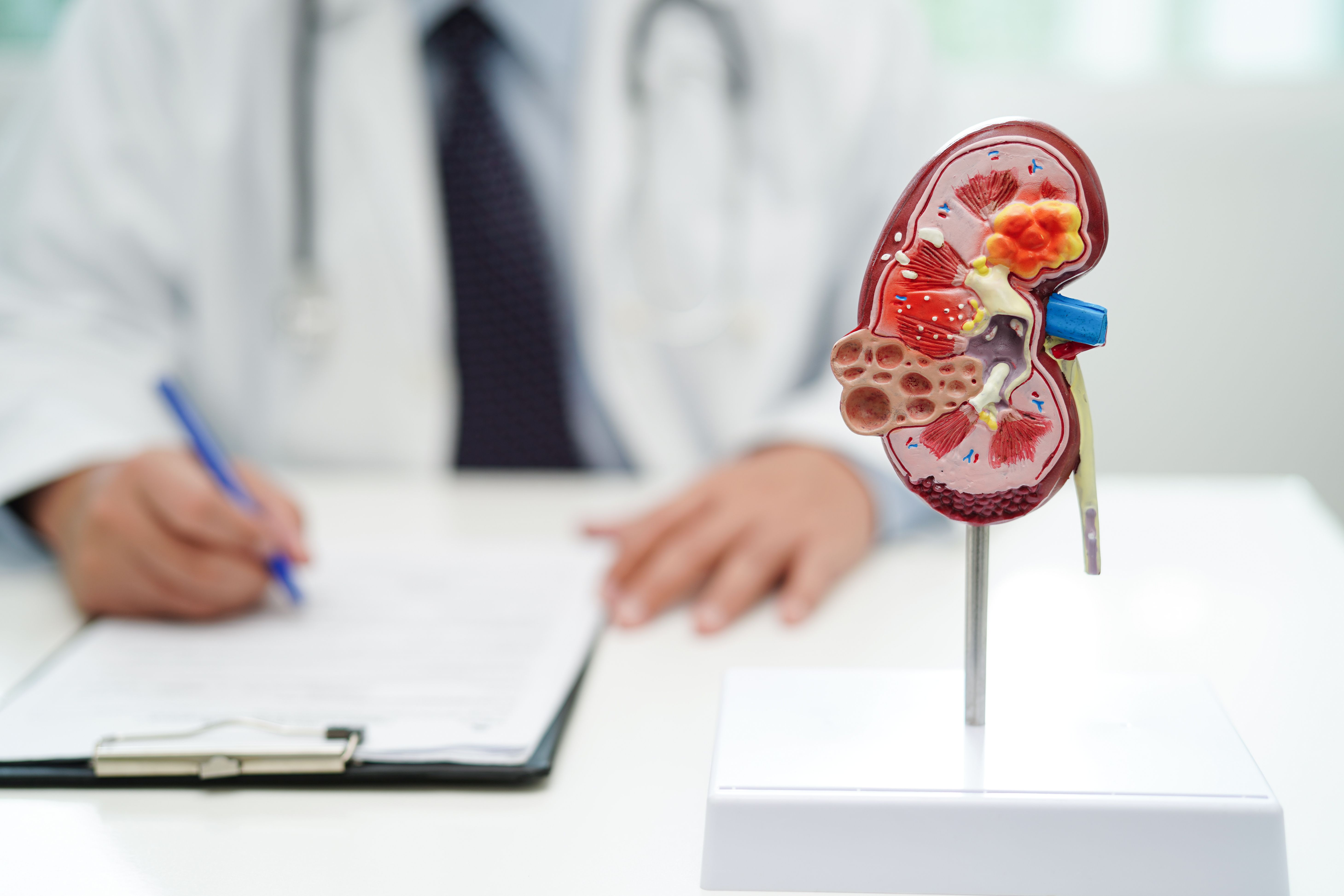
Chronic Kidney Disease
Latest News
Latest Videos

CME Content
More News

The treatment's expanded indication for chronic kidney disease (CKD) is expected to be available by April 2025.
Additional risk factors included older age and advanced chronic kidney disease (CKD) stage.
According to Pranav Garimella, pharmacists are essential in counseling patients with CKD on the appropriate use of semaglutide.

Semaglutide has been approved for reducing kidney disease progression and cardiovascular risk in patients with type 2 diabetes, marking a significant advancement in CKD management.

Clinical decision support systems based on algorithms have improved outcomes and reduced costs in other health care settings.
The authors suggest future research should consider inflammation and the gut as potential therapeutic targets improve depression in patients with chronic kidney disease (CKD).
Reductions in cerebellar volume were associated with cognitive deficits and lower kidney function in those with CKD.

These findings can lay the foundation for the development of machine learning models in chronic kidney disease (CKD) and kidney failure.
ABO-101 is supported by preclinical data that demonstrated significant reductions in urinary oxalate in PH1 disease models.

Some risk factors for chronic kidney disease (CKD) in patients with lupus nephritis (LN) and systemic lupus erythematosus (SLE) were renal impairment, delayed diagnosis, and hypertension.

Statin use is not associated with a change in incident chronic kidney disease (CKD) and estimated glomerular filtration rate in adult patients aged 65 and older.
The authors note that transferrin saturation levels may be a modifiable risk factor of chronic kidney disease (CKD) progression and all-cause mortality.

The new indication for semaglutide adds to the long list of conditions that the GLP-1 receptor agonist has been approved for, and provides a new option for high-risk patients with these chronic conditions.
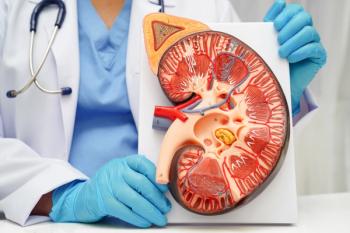
The reduction in cardiovascular end points and the increase in adverse events observed in a clinical trial were largely transportable to trial-eligible chronic kidney disease (CKD) populations from clinical practice.
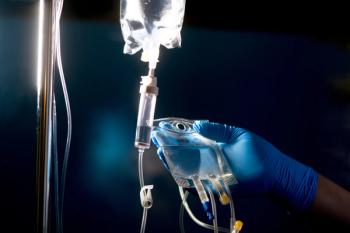
Patients with methotrexate (MTX)-induced acute kidney injury treated with glucarpidase were 2.70-times more likely to have kidney recovery and recover faster.

The Kidney Disease: Improving Global Outcomes guidelines can provide pharmacists with approaches to minimizing chronic kidney disease progression and complications.

The observational study shows a positive correlation between smoking and chronic kidney disease (CKD) whereas the analyses imply smoking-related covariates may be a factor.

Early diagnosis of chronic kidney disease (CKD) can help optimize treatment and manage the disease to prevent further decline.

Pharmacists can educate patients on controlling risk factors and stay up-to-date on emerging therapies like SGLT2 inhibitors and finerenone that can slow disease progression.
Results demonstrating safety and efficacy in those with anemia in non-dialysis dependent chronic kidney disease and anemia in dialysis-dependent chronic kidney disease were presented at the 2024 American Society of Nephrology Kidney Week.
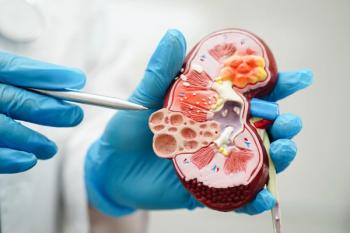
Zaltenibart could be an effective treatment option for C3 glomerulopathy as the most proximal inhibitor of the alternative pathway.
The results validate the protective benefits of the sodium–glucose cotransporter-2 inhibitors on renal function for those with type 2 diabetes.

Although disease screening reached high-risk patients, uptake was low, necessitating additional incentive to participate in voluntary testing.
Full traditional approval follows accelerated approval of the medication last year.
Pegcetacoplan is a targeted C3 therapy that is intended to regulate excessive activation of the complement cascade.