
Ron Lanton outlines enforcement uncertainty and downstream effects on biosimilars, specialty drugs, and pharmacy supply chains under most favored nation pricing proposals.

Ron Lanton outlines enforcement uncertainty and downstream effects on biosimilars, specialty drugs, and pharmacy supply chains under most favored nation pricing proposals.

Explore the profound connection between emotions and oncology pharmacy, highlighting the importance of acknowledging feelings in patient care.
These negative effects were primarily presented when cells were stressed by inflammatory proteins in patients with chronic kidney disease (CKD) with APOL1 mutations.

The FDA approves a groundbreaking 1-minute HIV self-test, enhancing accessibility and empowering individuals to manage their health discreetly.


Explore how targeted therapies and immunotherapies transform oncology, highlighting novel antibody treatments and their impact on patient care and outcomes.

Steven Pipe, MD, highlights the growing importance of pharmacists in hemophilia care as new therapies expand treatment complexity and require specialized expertise.
Flu vaccination during pregnancy safeguards maternal and infant health, reducing severe illness risks and providing essential immunity to newborns.
Research reveals that statins and metformin enhance survival rates in early-stage triple-negative breast cancer, offering hope for affordable treatment options.

Canomiks enhances consumer trust in the supplement industry through WhatToTrust, a platform evaluating product safety, efficacy, and scientific validation.

CVS expands its footprint by acquiring 626 Rite Aid and Bartell locations, enhancing pharmacy access amid industry challenges.

Research reveals the impact of SSRIs on pregnancy, highlighting risks and benefits for maternal mental health and fetal development.
In a Swedish cohort, young women with symptomatic long COVID were found to be at higher risk of postural orthostatic tachycardia syndrome (POTS), conferring additional burden.

Practice-based medical writing certificate programs offer tools to succeed in writing and beyond.

GLP-1 receptor agonists show potential as innovative treatments for alcohol and substance use disorders, addressing critical public health needs.

Emily Griffin is leveraging her social media platform to redefine pharmacy practice.
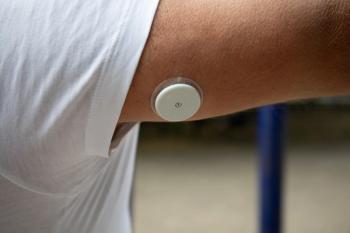
Primary care clinics enhance diabetes management by implementing continuous glucose monitors, improving access to essential care for patients nationwide.

Ron Lanton explains that increased pharmaceutical tariffs could drive up costs, reduce margins, and strain pharmacy supply chains, with few mitigation options available.

ACIP updates COVID-19 vaccine guidance, emphasizing shared decision-making, while addressing safety concerns and access issues for patients and pharmacists.

Pharmacists emphasize the importance of RSV awareness, testing, and vaccination for older adults and high-risk infants to enhance community health.
TACTI-004 follows 2 clinical trials, TACTI-002 and INSIGHT-003, which also assessed efti with pembrolizumab in first-line treatment of non–small cell lung cancer (NSCLC).

Patients must fight to be heard; health care providers are uniquely positioned to listen.

The approved indication now includes adults with moderately to severely active ulcerative colitis and Crohn disease who have not been treated with tumor necrosis factor blocking agents.
The move emphasizes the need for vigilant monitoring.

Bariatric vitamins ensure nutrient absorption for optimal recovery and health post surgery.
Higher levels of cyclic adenosine monophosphate (cAMP) may be key to diagnosing asthma and its severity as well as monitoring patients.
The ratio of high-density lipoprotein cholesterol (HDL-C) to low-density lipoprotein cholesterol (LDL-C) can predict cardiovascular disease (CVD) risk in patients with and without diabetes.

CDC layoffs threaten public health surveillance, prompting urgent reinstatement of staff as pharmacists face challenges in community health response.

Each month, Timothy Ulbrich will share lessons on mindset, strategy, and habits to help pharmacists achieve lasting security and financial freedom.