
Further, their status was evaluated at 14, 21, and 28 days after treatment, and at each point, the numbers of hospitalization were significantly lower in the treated group.

Further, their status was evaluated at 14, 21, and 28 days after treatment, and at each point, the numbers of hospitalization were significantly lower in the treated group.
According to the CDC, immunization against deadly diseases saves 2 million to 3 million lives per year.

The investigators, from Washington University School of Medicine in St. Louis, note that the responses measured were one-third as strong as those mounted by healthy individuals.
The FDA has also converted the indication for pembrolizumab from an accelerated to a full, regular approval for patients with urothelial carcinoma.

FDA’s green light for biopharmaceutical company’s drug candidate builds upon previous approval to treat pancreatic cancer.

The Pharmacy Times® Pharmacy Focus podcast provides the latest industry news and information, thought-leader insights, clinical updates, patient counseling tools, and innovative solutions for the everyday practice and business of pharmacy.

Pharmacy Times spoke with Ilisa Bernstein, APhA senior vice president, Pharmacy Practice and Government Affairs, about the latest FDA approval of the Pfizer-BioNTech COVID-19 vaccine.
Because patients may interact with a pharmacist more regularly than other health care providers, it is possible to detect potential signs and symptoms of atrial fibrillation in a patient during their encounters.
Atopic dermatitis treatments include creams for itching, calcineurin inhibitors such as Protopic and Elidel, as well as biologics such as Dupixent.
Brigham and Women’s Hospital conducted an analysis to measure the response of T cells from MMR and Tdap vaccines that could help to decrease COVID-19 severity.
Experts from Emory Healthcare and Winship Cancer Institute discuss the management of oncology patients by pharmacists within an NCI-designated comprehensive cancer center.
Doug Long, vice president of industry relations at IQVIA, and Scott Biggs, director of supplier services, discussed their session at the 2021 National Association of Chain Drug Stores (NACDS) Total Store Expo titled "Pharmaceutical Trends, Issues, and Forecasts."

How would you answer these patients' questions?
St. Jude Children’s Research Hospital reminds parents about the importance of getting the HPV vaccination during National Immunization Awareness Month.

In an interview with Pharmacy Times®, Guillermo Torre-Amione, MD, PhD, FACC, the chairman of Cardiol Therapeutics, discusses the potential use for CBD in hospitalized patients with COVID-19, as well as its therapeutic benefits in certain patients with cardiovascular disease.

Patricia C. Kienle, RPh, MPA, BCSCP, FASHP, director of Accreditation and Medication Safety at Cardinal Health and member of US Pharmacopeia’s (USP) Compounding Expert Committee, and Nicole Palmer, the senior manager of USP Volunteer Operations, discuss USP’s volunteer program.
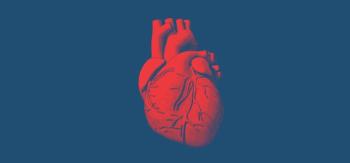
The AHA worked with a team of leading health authorities to develop the advisory, which highlights recommendations, algorithms, and guidance for health care professionals and researchers who specialize in heart and brain health.
It is currently debated whether or not sluggish cognitive tempo—defined as a collection of symptoms that include persistent dreaminess, fatigue, and slow working speed—is part of ADHD or separate from it.
Investigators from Oregon Health and Science University (OHSU) said that it is crucial to emphasize the importance of vaccinations, as well as the maintenance of public health measures that cut off the spread of the virus.
New paper shows that pregnant women often have low iron levels, while 1 in 4 have a severe deficiency.

Doses of the influenza live, intranasal (Flumist Quadrivalent, AstraZeneca) vaccine are now available in the United States in time for the 2021-2022 influenza season.

Pharmacists can step up counseling and advocate for best practices for children with chronic illnesses.

Investigators found that 65% of patients with cancer and COVID-19 were hospitalized and 17% required admission or transfer to a higher level of care.
Pharmaceutical companies continue trial for GBP510 in conjunction with GSK’s pandemic adjuvant.
Approved indications for idelalisib include relapsed chronic lymphocytic leukemia, small lymphocytic lymphoma, and follicular B-cell non-Hodgkin lymphoma.

Patients should be encouraged to remind their primary care provider to make sure they are on schedule and do not miss any vaccinations based on their age and conditions.

Study findings could influence how drug developers consider how opioid drugs cause tolerance and respiratory depression, and it suggests an alternative approach to developing safer analgesics.

Pharmacists carry a unique responsibility in the battle that has claimed 500,000 lives in the past 2 decades.

Pharmacy Times spoke with Dr. Trishia Shaw, the Clinical Assistant Professor of Pharmacy Practice at Chicago State University College of Pharmacy, on her presentation at the NPhA conference titled “Who’s your Provider”—Updates in Provider Status for Pharmacists.

Although the average demand for tocilizumab increased by 279% during the study period, fill rates for orders by hospitals dropped from 99% to 45%.