
Members, as well as health plans, pharmacy benefit managers, plan sponsors, and reinsurers want to know how to fund these expensive treatments to ensure positive outcomes.

Members, as well as health plans, pharmacy benefit managers, plan sponsors, and reinsurers want to know how to fund these expensive treatments to ensure positive outcomes.

Sarah Temkin, MD, of the Office of Research on Women’s Health at the National Institutes of Health, discusses some key points for pharmacists on the impact of biological and sociological variables on treatment outcomes.

Brought to You by The Sassy Pharmacist

Tom Hanzel, PharmD, MBA, discussed how vaccine mandates impact staffing shortages in long-term care facilities.

Ismail Lourido Ali, JD, director and counsel of policy and advocacy at the Multidisciplinary Association for Psychedelic Studies (MAPS), discusses whether the adoption and acknowledgement of psychedelic medicine in health care systems looks likely for the future.
Chemotherapy-induced nausea and vomiting can impact patients’ treatment outcomes.
According to a Kaiser Permanente research study, individuals who received COVID-19 vaccination had lower non-COVID-19 death rates than individuals who were unvaccinated.

Factitious disorder is not the same as inventing medical problems for practical benefit, such as getting out of work or winning a lawsuit.
Infectious Disease Society of America and Society for Healthcare Epidemiology of America make 3 new treatment recommendations for adults.
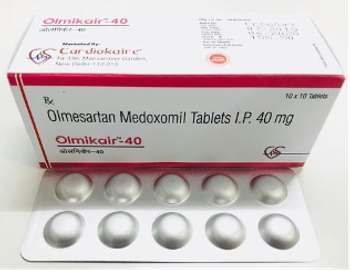
Benicar (Olmesartan Medoxomil) is indicated for the treatment of hypertension.

Tom Hanzel, PharmD, MBA, of Parata Systems, discussed challenges in pharmacy workflow and how automation could offer solutions.

They can create innovative face-to-face and virtual medication therapy management practices.
Luciano Costa, MD, PhD, of the University of Alabama at Birmingham, discusses the next steps for clinical research assessing daratumumab, carfilzomib, lenalidomide, and dexamethasone in patients with newly diagnosed multiple myeloma.

Sarah Temkin, MD, associate director for clinical research in the Office of Research on Women’s Health (ORWH) at the National Institutes of Health (NIH), discusses some of NIH ORWH’s upcoming goals and projects on the horizon that may impact the pharmacy field.

Ismail Lourido Ali, JD, director and counsel of policy and advocacy at the Multidisciplinary Association for Psychedelic Studies (MAPS), discusses which environments each psychedelic medicine is likely to be administered.

More transparent drug pricing could improve patient care.

Pharmacotherapy is modestly effective for short-term use in conjunction with healthy sleep habits and cognitive behavioral therapy.
Ten quiz questions to assess your knowledge on dialysis.
Fuchs' dystrophy causes fluid build up in the cornea, resulting in swelling.
Sarah Temkin, MD, associate director for clinical research in the Office of Research on Women’s Health at the National Institutes of Health, discusses how clinical trials have evolved to address biological and sociological variables in patient health.

Insulin glargine-yfgn (Semglee; Viatris) presents the first opportunity for retail pharmacists to dispense a biosimilar to patients.

The FDA looks to address a lack of diversity in cancer drug trials.

Luciano Costa, MD, PhD, of the University of Alabama at Birmingham, discusses how the results of the trial assessing daratumumab, carfilzomib, lenalidomide, and dexamethasone in patients with newly diagnosed multiple myeloma may impact the treatment landscape.
The Prosacea Medicated Rosacea Gel is used to treat rosacea, redness, and acne.
Ismail Lourido Ali, JD, director and counsel of policy and advocacy at the Multidisciplinary Association for Psychedelic Studies (MAPS), discusses which psychedelic drugs are currently under investigation for potential FDA approval.

Andrew Mulcahy, PhD, a senior policy researcher at RAND Corporation, discusses a recent report that showed the drug shortages in the United States may not be associated with problems in the global supply chain.
Laura Lee Hall, PhD, president of the Center for Sustainable Health Care Quality and Equity (SHC), addresses how the diabetes DRIVE program compares with the SHC DRIVE models for flu and COVID-19 vaccinations.
Study indicates those who experienced a greater amount of stress from the COVID-19 pandemic had reduced information processing speed.
Chronic hypersensitivity pneumonitis is a long-term lung condition in which the interstitial tissue surrounding the alveoli of the lungs becomes inflamed and develops fibrosis.
Foot drop is a sign of an underlying neurological, muscular, or anatomical problem.