
Adalimumab-aacf (Idacio) is indicated for chronic conditions such as rheumatoid arthritis, plaque psoriasis, ulcerative colitis, psoriatic arthritis, hidradenitis suppurativa, and juvenile idiopathic arthritis.


Adalimumab-aacf (Idacio) is indicated for chronic conditions such as rheumatoid arthritis, plaque psoriasis, ulcerative colitis, psoriatic arthritis, hidradenitis suppurativa, and juvenile idiopathic arthritis.
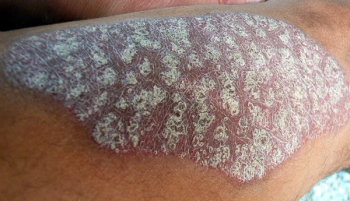
A modified secukinumab dosing regimen produced outcomes on par with currently recommended intervals in patients with moderate-to-severe psoriasis, but longer follow-ups are needed to confirm the findings.
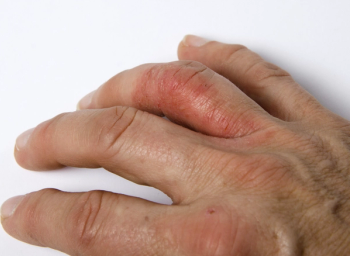
Guselkumab (Tremfya) is a fully human selective IL-23 inhibitor therapy FDA-approved for the treatment of adults with active psoriatic arthritis and with moderate to severe plaque psoriasis.

Research shows that patients with a severe form of psoriasis have a higher risk of death from cardiovascular disease.

The ability of an anti-interleukin-23 antibody to bind to key immune system components may help neutralize a key driver of inflammation right at its cellular source.

Ustekinumab is the first and only biologic that targets both cytokines interleukin (IL)-12 and IL-23.

Tapinarof (Vtama) cream, 1% is an aryl hydrocarbon receptor agonist indicated for the topical treatment of plaque psoriasis in adults.

People living with psoriatic arthritis deserve to be informed about ways to approach disease management holistically and with a personalized approach.

The study results support a hypothesis for a differentiated mechanism from risankizumab, the company says.
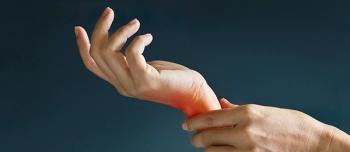
Agency greenlights secukinumab’s use for pediatric Treatment of enthesitis-related arthritis and Juvenile psoriatic arthritis.

Abatacept is also approved for adults with moderate to severe rheumatoid arthritis, active psoriatic arthritis, and moderate to severe polyarticular juvenile idiopathic arthritis for children 2 years of age and older.
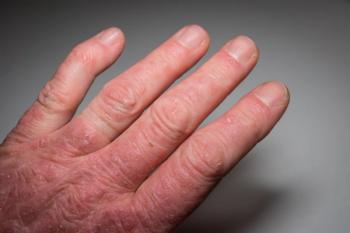
The safety profile observed in patients with active psoriatic arthritis administered upadacitinib was consistent with the safety profile observed in patients with rheumatoid arthritis.

Apremilast is indicated for the treatment of psoriatic arthritis, plaque psoriasis, and oral ulcers associated with Behçet disease.

The investigators concluded that a definite statement on risk or management recommendations cannot be made based on currently published data.

Risankizumab-rzaa (Skyrizi, AbbVie) is approved for the treatment of moderate-to-severe plaque psoriasis among adults who are candidates for systemic therapy or phototherapy.

Guselkumab also showed promising results in the treatment of psoriatic arthritis, with improved disease activity in joints and across multiple domains through 1 year.
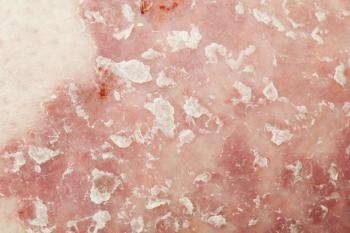
This is the first approval for secukinumab in a pediatric population in the United States for severe plaque psoriasis.
The POETYK PSO-1 and POETYK PSO-2 trials, which evaluated deucravacitinib 6 mg once daily, met both co-primary endpoints versus placebo, with significantly more patients achieving Psoriasis Area and Severity Index (PASI) 75 response and a static Physician's Global Assessment score of clear or almost clear (sPGA 0/1) after 16 weeks of treatment with deucravacitinib.

Some drugs commonly used for psoriasis can increase the risk of cardiovascular diseases, whereas other treatments can reduce the risk.

According to the study authors, this could lead to finding the exact molecular trigger and gives hope for developing a targeted PsA treatment in the future.

The FDA has approved golimumab for the treatment of patients 2 years of age and older with active polyarticular juvenile idiopathic arthritis.
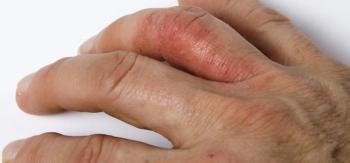
A virtual symposium held in conjunction with the Asembia Specialty Pharmacy Summit focused on psoriatic arthritis.
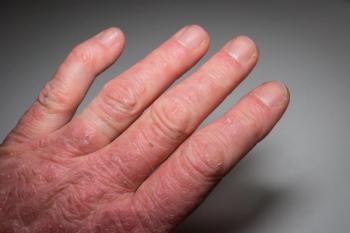
Secukinumab provided early and sustained resolution of enthesitis in patients with psoriatic arthritis based on post hoc analysis pooled data from the FUTURE trial.
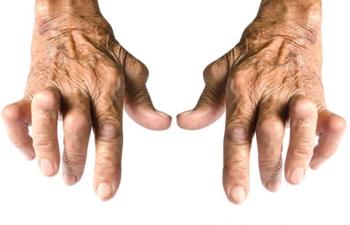
It is currently uncertain to what extent interleukin inhibitors may increase the risk of serious infections and cancer in patients being treated for rheumatic diseases.
Top news of the day across the health care landscape.