
A study of methotrexate in patients with psoriasis found that the immunosuppressive drug was more effective in patients without psoriatic arthritis than those with the added condition.

A study of methotrexate in patients with psoriasis found that the immunosuppressive drug was more effective in patients without psoriatic arthritis than those with the added condition.
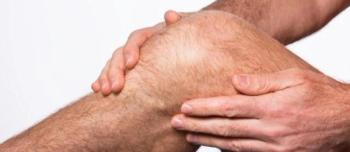
Phase 2b study results show durable responses rates and symptom improvement in patients with psoriatic arthritis.

The updated label includes new evidence that the treatment significantly impedes the progression of joint structural damage at 24 weeks compared to placebo for patients with active psoriatic arthritis.

The label update includes new evidence that the treatment significantly impedes the progression of joint structural damage at 24 weeks compared to placebo for patients with active psoriatic arthritis.
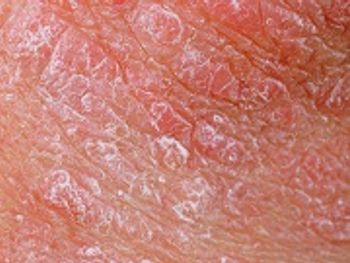
Risankizumab shows promise as an important treatment option for people living with plaque psoriasis.

Ron Lanton III, Esq, President, True North Political Solutions, makes predictions about how dealings with biosimilars may change in 2018.

Tofacitinib (Xeljanz/Xeljanz XR) improves disease severity for patients with psoriatic arthritis.
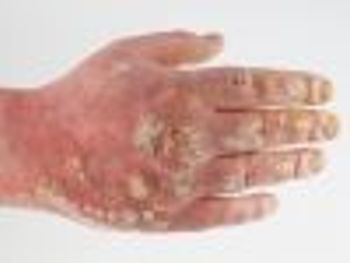
Up to 90% of patients with psoriasis develop plaque on their nails, palms of the hands, and soles of the feet.
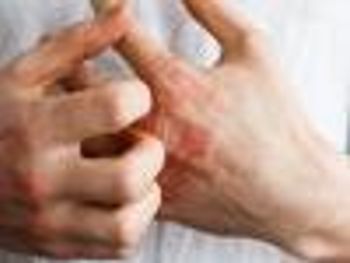
Hay fever and asthma are dependent on allergens that stimulate the immunoglobulin E pathway, which is not the case with atopic dermatitis.
Ixekizumab found to provide significant improvement in joint symptoms for patients not previously treated with a biologic disease-modifying antirheumatic drug.
Patients with moderate-to-severe nail psoriasis treated with secukinumab experienced significant improvement in the Nail Psoriasis Severity Index.
Secukinumab (Cosentyx) treatment observed to improve the signs and symptoms of psoriatic arthritis.

New Drug Applications filed for halobetasol propionate and tazarotene lotion, a topical treatment for plaque psoriasis.

Treatment for psoriatic arthritis may impact symptoms and patient-reported outcomes.
Patients with active psoriatic arthritis experience significant work instability based on disease activity.
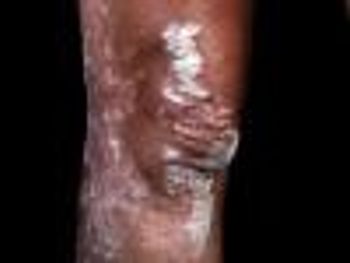
Several modalities found to be effective in the triage of psoriasis patients with musculoskeletal symptoms, according to a study presented at the 2017 American College of Rheumatology meeting.

Risankizumab treats moderate to severe chronic plaque psoriasis.

Calcipotriene and betamethasone dipropionate treats mild to moderate plaque psoriasis in adult patients.
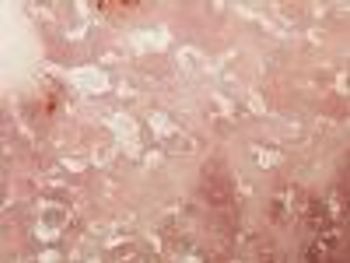
Two supplemental biologics license applications granted to golimumab to treat adults with psoriatic arthritis and adults with active ankylosing spondylitis.
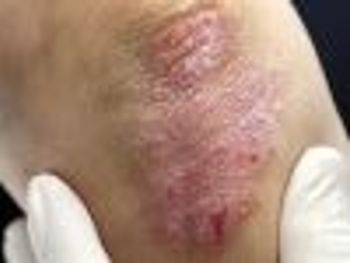
Ustekinumab (Stelara) treats moderate to severe plaque psoriasis in adolescents who are candidates for phototherapy or systemic therapy.
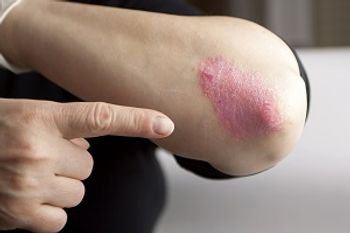
A fire ant toxin may lead to new medications for psoriasis.

For patients with psoriasis and/or psoriatic arthritis, actual-to-expected dosing ratios and costs were lower for etanercept than for ustekinumab.
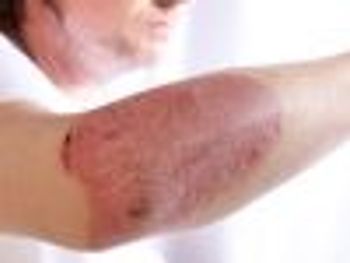
Findings may lead to future treatments for patients with psoriasis and psoriatic arthritis.

Top news of the week from Specialty Pharmacy Times.

Research finds that a robust pipeline of PsA agents will put pressure on Cosentyx.