Psoriatic Arthritis
Latest News

Latest Videos

CME Content
More News
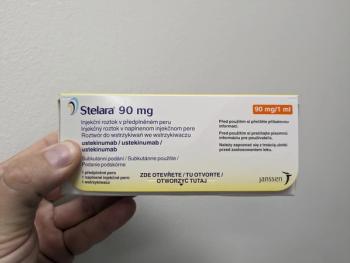
A new biosimilar to ustekinumab (Stelara) has been approved by the FDA, further expanding treatment options for patients with immune conditions such as plaque psoriasis and psoriatic arthritis.
Results of the POETYK PsA-2 trial indicate deucravacitinib’s effectiveness at reducing the severity and burden of symptoms in patients with psoriatic arthritis.
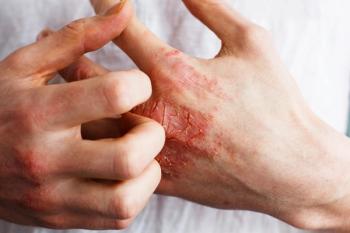
Through frequent interactions, pharmacists can discuss patients' treatment satisfaction, adherence issues, or potential adverse events.

Pharmacists play a significant role in managing gout by educating patients about the disease and ensuring proper medication adherence to urate-lowering therapy.

Ustekinumab-stba (Steqeyma; Celltrion) is a subcutaneous treatment for adult and pediatric patients with plaque psoriasis arthritis, psoriatic arthritis, Crohn disease, and ulcerative colitis.

If approved, the new indications include children 6 years and older with moderate to severe plaque psoriasis and children 5 years and older with active juvenile psoriatic arthritis.

The biosimilar is approved for the treatment of Crohn disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis.
The FDA previously accepted the biologic license application for CT-P13 as a subcutaneous formulation.
Approved with 3 new indications, bimekizumab-bkzx is the first and only interleukin-17 inhibitor approved for these diseases.

Ustekinumab-ttwe (Pyzchiva; Sandoz) is approved for all of the same indications as Stelara.

Investigators compared the reference products of adalimumab (Humira; AbbVie) and etanercept (Enbrel; Amgen) with biosimilar products.
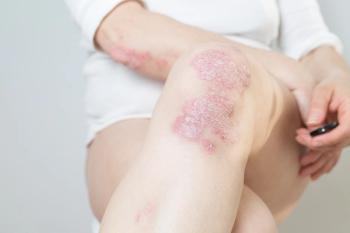
Ustekinumab (Stelara; Janssen Immunology) is a human monoclonal antibody that treats immune-mediated diseases such as psoriasis and psoriatic arthritis.

The 3 bimekizumab indications include psoriatic arthritis, non-radiographic axial spondyloarthritis, and ankylosing spondylitis.

In 2024, there is an urgent need for policies that will accelerate biosimilar use to achieve lower system-wide health care costs.
Adalimumab-aqvh (Yusimry; Coherus Biosciences) entered the market in July 2023, following approval by the FDA in December 2021.

The sBLA was based on data from a clinical study assessing the pharmacokinetics of switching between adalimumab and adalimumab-bwwd for patients with moderate to severe plaque psoriasis.

TAK-279 is an investigational oral allosteric tyrosine kinase 2 inhibitor for active psoriatic arthritis.
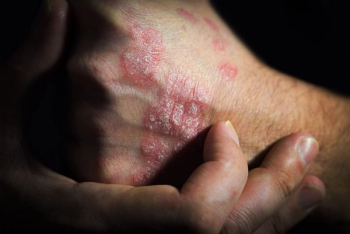
The biosimilar, now legally available at the pharmacy level, demonstrated similar safety, purity, and potency to the reference product.

The approval is supported by safety data from clinical trials assessing abatacept in the treatment of patients aged 2 to 17 with psoriatic arthritis.

New products and medications can be used as single agents or in combination with other agents for optimal treatment outcomes.

The intravenous formulation of secukinumab is administered in monthly 30-minute, weight-based dosing and requires no pre-medication or lab monitoring.
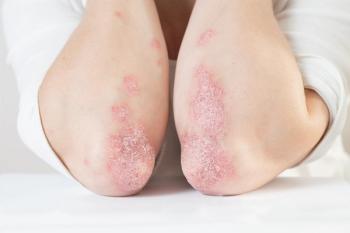
Previously, upadacitinib improved symptoms through 2 years in the SELECT-PsA 1 trial for those with psoriatic arthritis with prior inadequate response or intolerance to ≥1 non-biologic disease-modifying antirheumatic drug.

It is the second anti-TNFα biosimilar approved for use in the US.

Adalimumab-adbm (Cyltezo Pen; Boehringer Ingelheim) is approved by the FDA as an interchangeable biosimilar to Humira.
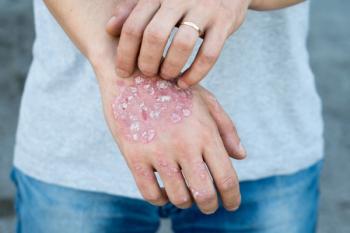
Due to the disease’s chronicity, the variability in treatment response and drug safety profiles, and the availability of multiple therapies, switching or discontinuing treatments is common in psoriasis.