
Temptation exists because more than 90% of these patients are prescribed opioid medications for pain.

Temptation exists because more than 90% of these patients are prescribed opioid medications for pain.

More than 53% of survey respondents reported having a diversion event within the last year, in addition to 37% who were aware of at least 1 colleague who has diverted controlled substances.

Researchers from the Mayo Clinic have found that inappropriate use of high-potency opioids has contributed to the opioid epidemic in the United States.

Why is this patient on oxybutynin if he does not have any problems with his bladder?

Cannabis products can counter some negative aspects of chemotherapy.
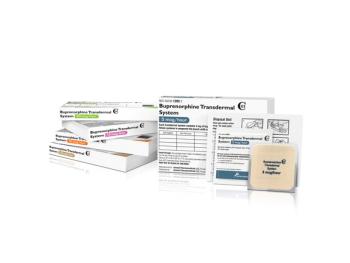
The FDA granted Amneal Pharmaceuticals for a generic version of buprenorphine (Butrans) transdermal system as well as a Competitive Generic Therapy designation.

Pain is the most common reason patients seek medical marijuana, yet patients' resulting consumption patterns of marijuana when treating pain may also have an effect on their health.
Research explores the impact of obesity on prescription opioid use, investigating specifically the link between obesity and long-term prescription use.

More than 90% of the legal marijuana products offered in medical dispensaries are much stronger than what physicians typically recommend for chronic pain relief via data from clinical studies, according to research published in PLOS ONE.

RedHill Biopharma Ltd today announced that it has completed the recently announced acquisition of the global rights to Movantik (naloxegol) for the treatment of opioid-induced constipation (OIC) from AstraZeneca.
A new study examining records from the Ohio Health Department from 2010 to 2017 found that white men between the ages of 30 and 39 had the highest risk for fatal opioid overdoses.

The research showed that the preventive effect of eptinezumab-jjmr was statistically significant versus placebo as early as day 1 and maintained throughout 3 months post-infusion.
The FDA has released a statement addressing the issue, stating that the organization is “not aware of scientific evidence connecting the use of NSAIDs, like ibuprofen, with worsening COVID-19 symptoms.”
Patients who received an opioid after tooth extraction reported higher levels of pain than patients who received a non-opioid.
Better, evidence-based treatments exist for this common condition, which can cause disability.

This approval marks the first for combination ibuprofen and acetaminophen for OTC use.

This is one of the first US population-based studies to compare overdose risk among 2 patient populations across an entire state.
In recent years, drugmakers have been accused of pushing opioid prescriptions on physicians across the United States and allegedly downplaying the risks of addiction. Meanwhile, governments feel that distributors and pharmacies have turned a blind eye to suspicious orders and failed to meet government requirements on painkillers.

The new drug is a nonopioid therapeutic option for patients experiencing moderate to severe pain.

Diclofenac sodium topical gel was first approved by the FDA in 2007 as a prescription drug, and was indicated for the relief of osteoarthritis pain in joints responsive to topical treatment, particularly the joints of the hands, knees, and feet.
To streamline the way opioid treatments are administered and to successfully treat addicts, a pharmacist’s clinical expertise is maximized in a medication-assisted treatment setting within opioid treatment programs.

Jeffrey Fudin, PharmD, DAIPM, FASHP, FFSMB, explains key facts pharmacists should know about cannabidiol products and pain management.

Published in the Journal of the American Medical Association, the study was conducted using a segmented regression analysis of an interrupted time series over a course of 30 months.

Hydromorphone prescription rates may explain the recent increase in cases of infective endocarditis among people who inject drugs.

The action marks the first and only OTC topical drug with cannabidiol to receive certification for its listing in the National Drug Code Directory, according to its manufacturer.