HIV
Latest News

Latest Videos
CME Content
More News

Fosamprenavir calcium (Lexiva ) is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.

Pharmacies can help bring inequities plaguing health care systems to an end to ensure the COVID-19 pandemic does not follow trends of inequity experienced in the HIV pandemic.
Ritonavir (Norvir) is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.

PQA is also committed to national goals related to HIV and reducing HIV ending the epidemic.
Symtuza, a 4-drug combination of darunavir (DRV), a HIV-1 protease inhibitor; cobicistat (COBI), a CYP3A inhibitor; and emtricitabine (FTC) and tenofovir alafenamide (TAF), both HIV-1 nucleoside analog reverse transcriptase inhibitors, is indicated as a complete regimen for the treatment of HIV-1 infection in patients who weigh at least 40 kg.
Prezista is an protease inhibitor indicated for the treatment of HIV-1 infection in patients aged 3 years and older.
It's extremely important for pharmacists to be involved in helping care for people who have HIV.

Ibalizumab-uiyk (Trogarzo, Theratechnologies) is an injectable CD4-directed post-attachment HIV-1 inhibitor for the treatment of HIV type 1 infection in heavily treatment-experienced adults.
There are other courses available on HIV, but PQA's surveys the 13 medication related quality measures out there for HIV.

The FDA previously placed a clinical hold on lenacapavir in borosilicate vials in all clinical studies because of emerging concerns about the compatibility of vials made of borosilicate glass.

After 14 months without ART, a patient with HIV only had transient detection of trace levels of HIV DNA in her blood cells after a stem cell transplant for acute myeloid leukemia.
Results of a meta-analysis of randomized clinical trials published in JAMA shows improvement in 6 of 8 domains.

Cabotegravir inhibits HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral DNA integration.
Letermovir has been approved by the FDA to prevent severe CMV in patients who are asymptomatic and undergoing allogeneic hematopoietic stem cell transplants.
PrEP use has been associated with lowering HIV infections worldwide, however use of the treatment alone is not sufficient to prevent HIV from spreading.

Cabotegravir is the only current HIV-1 PrEP medication that does need to be taken daily.
A panel of experts discuss the role of PrEP in HIV prevention with a focus on current and newly approved options.

Triumeq is a tablet containing 50 mg of dolutegravir, 600 mg of abacavir, and 300 mg of lamivudine.

FDA approves expanded indication for the treatment of HIV-1 in virologically suppressed adolescents 12 years of age or older, who weigh at least 35 kg, and who are on a stable antiretroviral regimen, with no history of treatment failure or known or suspected resistance to either cabotegravir or rilpivirine.
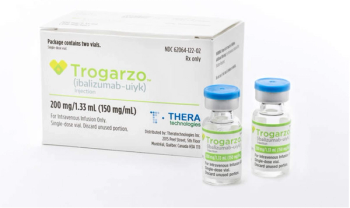
Ibalizumab-uiyk (Trogarzo) is a CD4-directed post-attachment HIV-1 inhibitor.

FDA approves label update for Cabenuva that makes the therapy’s oral lead-in with cabotegravir and rilpivirine tablets optional.

The FDA recently approved cabotegravir 200 mg/mL and rilpivirine 300 mg/mL as a once-monthly treatment for adults with HIV-1 who are virologically suppressed on a stable antiretroviral regimen.
Moderna is currently evaluating 2 HIV vaccine strategies that involve germline targeting and immune-focusing approaches.
Enhanced tools in the field of structural biology may reveal new insights into HIV that could lead to a vaccine to protect against the virus progessing to AIDS.

New strategies are needed to increase HIV prevention and testing efforts among high-risk communities.





























