Hematology
Latest News

CME Content

Ligand and Spectrum Pharmaceuticals Announce Specialty Formulation of Melphalan for Multiple Myeloma
Using Captisol technology, scientists have developed a more stable formulation of melphalan that may facilitate higher-intensity regimens in patients with multiple myeloma undergoing autologous stem cell transplant procedures.
A variety of treatments to counteract the bone-wasting effects of multiple myeloma are in development.
Early results of a phase-1/-2a indicate that an infused monoclonal antibody treatment may target the abnormal cells associated with multiple myeloma while stimulating an immune response.
Study finds significant racial and geographic disparity in prevalence of monoclonal gammopathy of undetermined significance.

In 2013, many new medications and new indications were approved.

Poorly controlled type 2 diabetes mellitus in patients with multiple myeloma (MM) may increase the risk of adverse cardiovascular outcomes, kidney damage, and peripheral neuropathy. Considering the effect of medications to treat MM on blood glucose control may be an important part of choosing a treatment regimen for some patients.

Promoting vaccination against Streptococcus pneumoniae can prevent meningitis and related complications.
Investigators strengthened the results of a 2012 trial with a long-term follow-up of an important phase III trial evaluating a bortezomib-based regimen versus a vincristine-based regimen.

Carfilzomib's ability to inhibit myeloma cell growth and ability to trigger a greater response in proteins associated with cell death than other therapies makes it a promising therapy for multiple myeloma patients.

In this OncLive video, Jatin J. Shah, MD, assistant professor in lymphoma/myeloma in the Division of Cancer Medicine at the University of Texas, MD Anderson Cancer Center, discusses novel therapeutics for the treatment of multiple myeloma.

Novartis announced a demonstrated improvement in progression-free survival in patients using LBH589 (panobinostat) with bortezomib and dexamethasone.
Drs. Jasielec and Jakubowiak review the state of the art in selecting an optimal induction therapy regimen for patients with multiple myeloma.

The FDA approved Imbruvica (ibrutinib) to treat patients with mantle cell lymphoma (MCL), a rare and aggressive type of blood cancer.
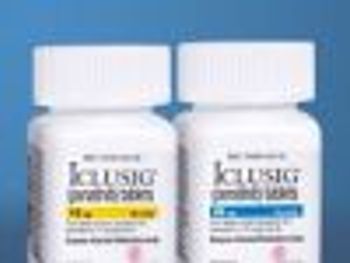
The FDA has asked the manufacturer of the leukemia chemotherapy drug Iclusig (ponatinib) to suspend marketing and sales of Iclusig because of the risk of life-threatening blood clots and severe narrowing of blood vessels.

Those concerned about different types of cancer should band together to support research into prevention, detection, and cure-and to counter the exorbitant expense of treatment.

Investigators in Spain have demonstrated that a regimen of lenalidomide and dexamethasone reduces the risk of disease progression and improves survival in patients with high-risk smoldering multiple myeloma.
Investigators in the Czech Republic have found an association between soft tissue lesions and diminished survival in patients with multiple myeloma.
After promising results from early studies, investigators have received approval to start testing daratumumab in patients with multiple myeloma.
Researchers highlighted the latest developments in the treatment of multiple myeloma at the 2013 ASCO Annual Meeting.
Patients with previously treated multiple myeloma responded well to therapy with the monoclonal antibody elotuzumab in combination with lenalidomide and low-dose dexamethasone, with a high objective response rate and longer progression-free survival (PFS) in those who received the dose being taken into phase III studies.
New therapies advance the treatment of this hematologic cancer.

New therapies advance the treatment of this hematologic cancer.

When giving antineoplastics to obese and morbidly obese patients, clinicians must be careful not to under- or overdose.
The FDA approved Pomalyst (pomalidomide) to treat patients with multiple myeloma whose disease progressed after being treated with other cancer drugs.