Diabetes
Latest News

Latest Videos

CME Content
More News

The t:slim X2 insulin pump and Tandem Mobi system both use Control-IQ.

Radon exposure during pregnancy was significantly linked to increased odds of gestational diabetes.
SkinTE could aid Wagner grade 1 diabetic foot ulcers by regenerating and activating the tissues surrounding the wound to heal it completely.
Diabetes management through insulin could play a significant role in preventing antibiotic resistance.

Age, body mass index, and male sex are also associated with the development of diabetes.

Diabetic macular edema (DME) is the leading cause of new blindness in the US.
Overall, there is no long-term increased risk of developing thyroid cancer after initiating treatment with a glucagon-like peptide-1 (GLP-1) receptor agonist, although there is a short-term increase.

Three experts from UPMC Health Plan highlight the importance of treating patients’ physical, mental, and social health when managing diabetes, rather than the disease alone.

The diabetes treatment landscape is rapidly evolving, with new combination therapies and oral GLP-1 medications, positioning pharmacists to have an important role in personalizing care.
Glucose monitoring can help providers and pharmacists optimize medication management for personalized diabetes care.
Jennifer Clements, a clinical professor and the director of pharmacy education at the University of South Carolina, discusses the pharmacists role in diabetes care.
Pharmacy Times interviews Jennifer Clements, a clinical professor and the director of pharmacy education at the University of South Carolina, about glucagon-like peptide-1 receptors for diabetes treatment.

Limited access to healthy food in redlined communities contributes to higher rates of chronic disease and reduced life expectancy.

Age, frequent snoring, hypertension, and poor glycemic control are associated with excessive daytime sleepiness.
However, the effects on patient-reported outcomes were inconclusive when compared with self-monitoring blood glucose or the usual care.

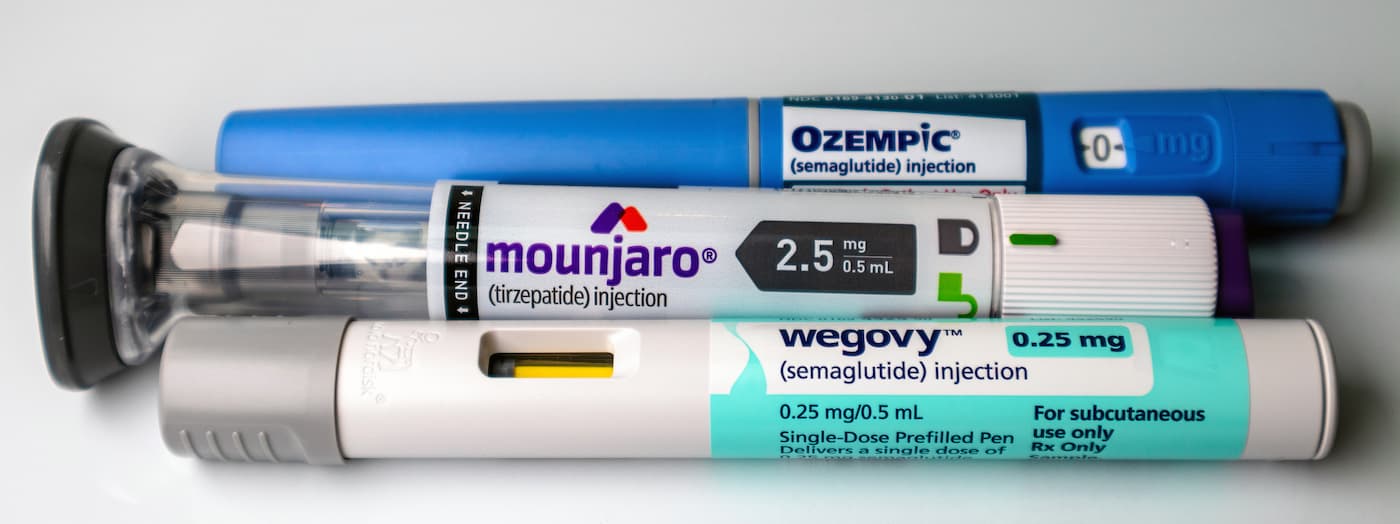
Despite the explosion of public interest in these drugs, ongoing shortages and debates over compounding made access difficult.

The approval of the generic to liraglutide injection, currently in shortage, could help increase patient access to the type 2 diabetes treatment.
Compounding pharmacies have a 60- to 90-day grace period to complete production as it continues to monitor supply and demand.

This could be attributed to improved diet and health awareness.

Ultra-processed food consumption fuels the rising rates of childhood obesity, diabetes, and liver disease, highlighting opportunities for pharmacist intervention.

Rural pharmacies and hospitals face unique challenges in maintaining adequate staffing and drug supply inventory to serve patients who often travel long distance.

Pharmacists play an essential role in educating patients about various health-related topics, including type 2 diabetes and nutrition.

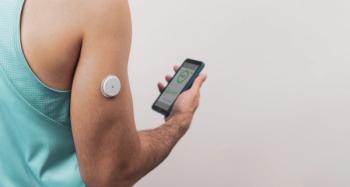
Pharmacists can leverage continuous glucose monitoring data to personalize diabetes management, overcome clinical inertia, and improve glycemic control for patients.
As use of injectable medications increases for diabetes and other indications, proper administration is crucial.

The pharmacist’s role includes determining if there are any medications that are contributing to magnesium deficiency for patients with diabetes who are at an increased risk.