
AWARxE is a free resource created by the National Association of Boards of Pharmacy to raise awareness on the dangers of prescription drug abuse.

AWARxE is a free resource created by the National Association of Boards of Pharmacy to raise awareness on the dangers of prescription drug abuse.


The FDA's first approval of a biosimilar drug offers new opportunities, but pharmacists need to remain alert for the opposition that might arise.
The FDA today approved a CPR device that the agency claims may improve a patient's chances of surviving cardiac arrest.


Improvements in nutrition, exercise, stress, and sleep may help patients with prediabetes reduce their risk of progressing to type 2 diabetes.

Digital health is making large strides due to the rapid development and incorporation of technology, and pharmacies are looking to capitalize on this.

The American Heart Association/American Stroke Association topped entries from 44 other countries to earn the World Stroke Organization's First Place "Gold Award" for World Stroke Day 2014.

A low sodium mineral salt product jointly developed by AkzoNobel and flavor experts Givaudan recently won a leading industry innovation award at the annual Global Food Technology & Innovation Summit in London.

The PERSIST-1 trial met its primary endpoint in the intent-to-treat population with statistically significant activity observed in patients irrespective of their initial platelet count, including patients with very low platelet counts at study entry, a condition known as severe or life-threatening thrombocytopenia.

The online video contest presented by the American Academy of Neurology and the American Brain Foundation helps raise awareness of the need for more funding and research into the prevention, treatment, and cures of brain diseases.
Hospira is voluntarily recalling a mislabeled lot of its injectable anti-seizure medication, magnesium sulfate in 5% dextrose, as well as 1 lot of its 0.9% sodium chloride injection, following a confirmed report of particulate in a single unit.

R. Jeffrey Hedges, president and CEO of RJ Hedges and Associates, talks about how non-compliance with Medicare Part B, C, and D standards can negatively affect a pharmacy's business.

An estimated 6 billion individuals worldwide had cold or flu symptoms in the past year, resulting in a collective total of 35 billion sick days.

Including often-ignored random blood glucose values in screening guidelines could help clinicians detect diabetes risk.
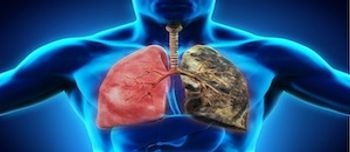
Leonard Nimoy's recent death from end-stage chronic obstructive pulmonary disease (COPD) brought the condition into the spotlight, and may bring patients with questions to the pharmacy counter.

An attentive CVS pharmacist alerted police to a prescription fraud case after a customer handed him suspicious-looking prescriptions that turned out to be forged.

Despite persistent concerns over inappropriate antipsychotic prescribing in children, a new study suggests the majority of prescriptions for the medications are issued appropriately.

Screening men with erectile dysfunction (ED) for cardiac risk factors could save the health care system $28 billion over a 20-year period.

Ludy L. Underwood, Cardinal Health sales manager for retail/alternative care, pharmaceutical division, talks about new programs Cardinal Health has introduced in response to industry changes.

Marc C. Thompson, executive coach and best-selling author, talks about strategies pharmacists can use to engage employees in pharmacy operations.

Do you know a student pharmacist who demonstrates extraordinary spirit and passion for the profession? Nominate him or her for the Next-Generation PharmacistTM 2015 Future Pharmacist award.

The FDA today approved isavuconazonium sulfate for adults with invasive aspergillosis and invasive mucormycosis.


The approval of the US's first biosimilar is an important step in increasing access to life-saving and life-improving biologics, but it is just a first step.

Several GPhA member companies have developed, manufactured and are marketing biosimilars around the globe.

The FDA recently approved 2 new antibiotic combinations targeting resistant Gram-negative organisms.

There's no guessing which team retired pharmacist Irving Rider is rooting for this March Madness.
The FDA today authorized the use of the first device approved to treat dialysis-related amyloidosis.

Sagent Pharmaceuticals is voluntarily recalling 6 lots of its atracurium besylate injection following FDA observations of practices at the manufacturer's site that could potentially affect the products' sterility.