This first-to-market point-of-sale platform combines industry expertise, artificial intelligence and comprehensive, deep-data libraries to seek out and identify errors so that the right discounts are applied to the right transaction.

This first-to-market point-of-sale platform combines industry expertise, artificial intelligence and comprehensive, deep-data libraries to seek out and identify errors so that the right discounts are applied to the right transaction.

This is the first FDA approval for an anti-PD-L1 therapy as part of a combination regimen for patients with advanced RCC, according to EMD Serono.

The lawsuit, filed in U.S. District Court for the District of Connecticut, alleges a broad conspiracy to artificially inflate and manipulate prices, reduce competition, and unreasonably restrain trade for more than 100 different generic drugs.

Amber Draper, PharmD, BCOP, clinical pharmacist, Winship Cancer Institute in Georgia discusses current recommendations for colon cancer screening. This video was filmed at the 2019 Hematology/Oncology Pharmacy Association Annual Conference in Fort Worth, Texas.

In addition to first-line treatment of blepharospasm, the drug is indicated for adult patients with cervical dystonia, upper limb spasticity, and chronic sialorrhea, or excessive drooling.

The FDA's approval was based on 6-month and 1-year results from the PANORAMA clinical trial.

Kaposi sarcoma is a cancer caused by an associated herpesvirus that develops from the cells that line lymph or blood vessels, and most commonly affects individuals with HIV.

The novel intravenous immune globulin (IVIG) product is indicated for individuals with primary humoral immunodeficiency disease (PIDD), which includes X-linked and congenital agammaglobulinemia, common variable immunodeficiency, Wiskott-Aldrich syndrome, and severe combined immunodeficiency.

Lisa Holle, PharmD, BCOP, FHOPA, associate clinical professor at the University of Connecticut, discusses recent key trials involving non-metastatic castrate-resistant prostate cancer.

JK is experiencing gastrointestinal effects. What could be the cause?

Novartis has issued a voluntary recall of 3 lots of Promacta (eltrombopag) 12.5 mg for oral suspension to the consumer level, due to a risk of potential peanut flour contamination that occurred at a third-party contract manufacturing site.

As The Pharmacy Sage, I often write about solving your most brutal problems by asking the right questions.

Mary Koslap‐Petraco, DNP, PNPPC‐BC, CPNP, FAANP, Stony Brook University School of Nursing, discusses the difference between active and passive immunizations, and how they work to prevent disease. This video was filmed at the 2019 National Association of Pediatric Nurse Practitioners (NAP NAP) annual meeting in New Orleans.

Pharmacists can counsel patients on the best ways to reduce the risk of skin cancer.

Patients with HCC who have an alpha fetoprotein (AFP) of ≥ 400 ng/mL and have been previously treated with sorafenib are eligible for this treatment.

The designation is intended to expedite the development and review of drugs for serious or life-threatening conditions when preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on one or more clinically significant endpoints.

The final guidance will provide clarity for developers who want to demonstrate that their proposed biological products meet the statutory interchangeability standard under the Public Health Service Act.
An agreement between the US Department of Health and Human Services and Gilead will provide HIV prevention drugs to uninsured individuals who are at risk.
Mylan Pharmaceuticals has received final approval from the FDA for its Abbreviated New Drug Application (ANDA) for Erlotinib Hydrochloride Tablets, 25 mg, 100 mg and 150 mg.
The House investigational hearing drew testimony from health care industry experts representing insurers, patient advocacy groups, and pharmaceutical companies.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.
The 17% drop is the biggest ever recorded in the United States market, according to the IQVIA Institute for Human Data Science.

Bernard Marini, PharmD, BCOP, an Inpatient Hematology Specialist at the University of Michigan, discusses the selection of therapies for patients with acute myeloid leukemia, during the Hematology/Oncology Pharmacy Association's 2019 annual meeting in Fort Worth, Texas.

Lawmakers said the generic and biosimilar manufacturers depend on the 2 databases to bring more affordable medications to the market.
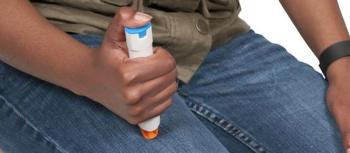
According to Sandoz, the company is working to make its epinephrine 0.3 mg and 0.15 injections available to the retail market, which would enable individuals to obtain this medicine at local pharmacies.

In announcing the requirement, Health and Human Services Secretary Alex Azar said Americans deserve to know the prices of the medications they receive.

Positive results were collected from a Phase 3 study of an investigational fixed-dose combination nasal spray for the treatment of seasonal allergic rhinitis (SAR), according to Glenmark Pharmaceuticals.

The national retail chain is also providing a more wholesome assortment of pet foods and the company is expanding in-store veterinary clinics.

Jaron Stout, PharmD, owner, consulting pharmacist, Collaborative Pharmacy Consultants, discusses the benefits of pharmacist services and a personal success story. This video was filmed at the American Society of Consultant Pharmacists' 2019 Forum in Washington, DC.

Suzanne Soliman, PharmD, founded the Pharmacist Mom Group on Facebook, and it has since grown to be 1 of the most active on the popular social media platform, with more than 2 million posts last year alone.