NKTR-181 is currently under review with the FDA, with a Prescription Drug User Fee Act (PDUFA) target action expected in August 2019.

NKTR-181 is currently under review with the FDA, with a Prescription Drug User Fee Act (PDUFA) target action expected in August 2019.

According to the ADA, best practice is to pack at least twice as much medications and glucose testing supplies as normally needed to minimize complications due to travel delays or lost luggage.3 Many manufacturers of insulin pumps will provide a loaner for international travel.

Calcipotriene foam was approved by the FDA in 2010 for patients age 18 years and older.

Norris Turner, PharmD, PhD, vice president of Strategic Alliances and Measures at PQA discusses the uses of PQA's measures in Medicaid during the Pharmacy Quality Alliance Annual Meeting in Baltimore, MD.

Regulatory affairs is another frontier for pharmacists to explore, and pharmacists are just scratching the surface of the opportunities in this area. In this interview with pharmacist Brendan Doran of CTI Clinical Trial & Consulting, he touches on the many phases of the drug life cycle with an emphasis on the process of bringing a drug to regulatory bodies for market approval, and gives a look into some of the current and emerging roles available in regulatory affairs.

Results of study demonstrate safety profile in pediatric patients.

The number of cases of the disease in 2016 was the highest since 1972 and represents a decades-long trend.

This ad was supported by Church & Dwight Co. Inc., featuring Feryal Hajee, MD.
The new phase 2 data for an investigational once-daily, fixed dose combination currently in development for the treatment of inadequately controlled asthma was presented at the 2019 Annual International Congress of the American Thoracic Society (ATS) in Dallas.

Laura Cranston, RpH, CEO of Pharmacy Quality Alliance (PQA), discusses the current landscape of medication quality during the PQA Annual Meeting in Baltimore, MD.

The acquisition agreement with Peloton includes the drug candidate PT2977, a novel oral HIF-2α inhibitor in late-stage development for renal cell carcinoma (RCC).

Solifenacin succinate tablet is a muscarinic antagonist indicated to treat this condition, with symptoms of urgency, urge urinary incontinence, and urinary frequency.

Lisa Holle, PharmD, BCOP, FHOPA, associate clinical professor at the University of Connecticut, explains the role of genomics in prostate cancer.
The drug is indicated for seizures that are distinct from a patient's usual seizure pattern, in individuals age 12 years and older with epilepsy.

Why is a woman's health declining, and she's turning yellow?

Parvus Therapeutics and Genentech have entered into a worldwide collaboration and licensing agreement for the development, manufacturing, and commercialization of treatments for inflammatory bowel disease, autoimmune liver diseases, and celiac disease using nanomedicines (Navacim) developed by Parvus.

Lisa Hines, PharmD, vice president of Measurement and Operations at Pharmacy Quality Alliance (PQA), discusses the alliance's new opioid measures and how PQA identified the need for them during the PQA Annual Meeting in Baltimore, MD.
Bruce Feinberg, DO, vice president and chief medical officer of Cardinal Health Specialty Solutions, recently explored both sides of the debate as to whether these tools are effective for managing costs or add barriers to patient access.

A goal of SBS treatment is to restore the patient’s ability to absorb nutrients and reduce long-term dependence on parenteral support.

In this video, sponsored by Cardinal Health, female pharmacists and pharmacy owners discuss what excites them about the future of pharmacy, including innovation, opportunity, and evolution of the industry.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

The final rule requires Part D plans to adopt tools that provide clinicians with information that they can discuss with patients on out-of-pocket costs for prescription drugs at the time a prescription is written.
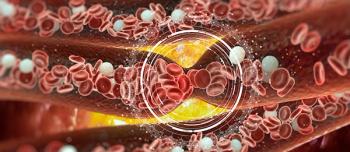
VTE can include deep vein thrombosis and pulmonary embolism, which can lead to death.

Lisa Hines, PharmD, vice president of Measurement and Operations at Pharmacy Quality Alliance (PQA), discusses PQA's new opioid measures and what they evaluate during the PQA Annual Meeting in Baltimore, MD.

When both of these compounds are in abundant supply, good things happen.

The annual KANTAR Readership Study encompasses readership habits of thousands of pharmacists and pharmacy professionals, working in the areas of chain, independent retail pharmacies and health-system pharmacies.
Civica has made plans to deliver 14 essential generic medications this year.

The combination therapy received Breakthrough Therapy designation in March 2019 for previously-untreated CLL.

Mary Koslap‐Petraco, DNP, PNPPC‐BC, CPNP, FAANP, Stony Brook University School of Nursing, discusses which vaccines that should be received by infants traveling outside the United States, and at what age they should be administered.
This report revealed that in 2016, an estimated 3900 of 4200 or 93% incident HIV infections among black women would not have occurred if the incidence for black women were the same as that for white women.