
Health system and hospital pharmacies could see more opportunities for revenue recovery for health system and hospital pharmacies in 2019, as well as increased transparency and access to care for patients.

Health system and hospital pharmacies could see more opportunities for revenue recovery for health system and hospital pharmacies in 2019, as well as increased transparency and access to care for patients.
In a 2-part study, a research team with Desert Regional Medical Center in Corona, CA is analyzing the need for pharmacist intervention for patients with HIV.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

In this clip, Jon Edwards, a director of marketing for Baxter, discusses the company's "Stand and Deliver" campaign.

Pharmacists must remain educated about errors and ways to prevent them, according to the Institute for Safe Medication Practices.

In this clip, Pharmacy Times' Ed Cohen interviews' IQVIA representative Miranda Rochol about the company's services for pharmacists.

We know that burnout continues to plague health care professionals.

In this clip, Athar Mirza, a senior director of marketing for Baxter, discusses the company's Stand and Deliver campaign.

As technological innovations continue to transform health care, many pharmacists and other providers are increasingly looking to digital solutions for improving medication adherence among their patients.

In a Tuesday session at the American Society of Health-System Pharmacists (ASHP) Midyear Clinical Meeting in Anaheim, CA, some predictions were shared from the ASHP Foundation’s Pharmacy Forecast.

Although smart pump alerts can be an useful tool in maintaining patient safety, it is important for health systems to take a collaborative and data-driven approach to reducing unnecessary alerts.

Just under one-fourth of all Americans have arthritis.

Are your business decisions putting money into your bank account?

In this clip, Erin McCreary, PharmD, BCPS, an antimicrobial stewardship/infectious disease clinical pharmacist at the University of Pittsburgh Medical Center, reflects on her experience of returning to Anaheim for ASHP Midyear 4 years after doing so as a student.
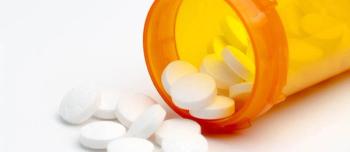
These products are being recalled due to detected trace amounts of an impurity, N-nitrosodiethylamine (NDEA) contained in the API valsartan.

As smart infusion technology is increasingly used by pharmacists in health-system settings, analyzing data from these devices can prove key to improving patient health.

The American Society of Health-System Pharmacists (ASHP) recognized its Donald Francke Medal recipient and other award winners at the 2018 ASHP Midyear Clinical Meeting in Anaheim, CA.

Safety data came from both phase 3 studies as well as a phase 2 study of Dextenza, which together totaled 351 patients.

The 2018 ASHP Midyear Clinical Meeting got off to a magical start today when basketball legend, businessman, and HIV/AIDS advocate Earvin “Magic” Johnson delivered the keynote address at the convention’s opening session, drawing upon his experiences, both on the basketball court and in the business world, to empower attendees to embrace their potential as health care providers and leaders.

In this clip, Mark Eastham, RPh, the senior vice president and general manager of McKesson RxO, discusses recent trends in health care delivery.

The research, a part of AmerisourceBergen’s first-ever Pharmacy Check-Up: Activity & Barriers to Care Analysis, examined various pharmacy settings.
Flu levels are typically at their highest between December and February.
In a satellite symposium at the 2018 American Society of Health-Systems Pharmacists (ASHP) Midyear Clinical Meeting in Anaheim, CA, the increasing rate of drug shortages was discussed, along with best practices pharmacists can use to mitigate the ongoing issue.

Rural areas are prone to unique health care challenges that are being met with policy changes and innovation.

Can you solve the pharmaceutical mystery? Each week, a new case study is presented.

Brock Slabach, MPH, FACHE, Senior Vice-President of the National Rural Health Association, discussed new developments in rural health care at the American Society of Health-System Pharmacists (ASHP) Midyear 2018 Clinical Meeting in Anahein, CA. In this clip, he discusses how Congress might support rural health care in 2019.
While scientists know more about the HIV infection than ever, and can prevent it from progressing into AIDS, there is still no known cure for the virus.

It might seem a bit odd to discuss ASHP Midyear before the holidays, but it’s never too soon to start looking ahead.

This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings, and more. Our Week in Review is a can't miss for the busy pharmacy professional.

ASHP’s 2018 Midyear Clinical Meeting is set to begin, and Pharmacy Times will be on site covering it.