
Erica Marchese, PharmD, MHA, BCPS, BCOP, BCSCP, discussed the revision of pharmacy and the contributing roles within the pharmacy team.

Erica Marchese, PharmD, MHA, BCPS, BCOP, BCSCP, discussed the revision of pharmacy and the contributing roles within the pharmacy team.

Penbraya (Pfizer Inc) is the first and only approved pentavalent vaccine that confers protection against the most common meningococcal serogroups—A, B, C, W-135, and Y, in individuals 10 through 25 years of age.
An overview of updated CDC ACIP recommendations for COVID-19 vaccines available through commercial channels for the 2023-2024 season.
Subcutaneous nivolumab had demonstrated noninferiority of Cavgd28, Cminss, and ORR compared to the intravenous form of treatment.
Women with HIV reported higher rates of physical abuse than men with HIV, but women had more social support from family and friends.
In a recent study, approximately 99% of children who received solid organ transplant and live vaccination against rubella had antibodies 1 to 3 months post-vaccination.
Study authors suggest that saliva samples can be a complementary testing method to provide additional information on pneumococcal carriage and serotypes that may be undetected by nasopharyngeal testing.

The statistically significant and clinically meaningful improvement in disease-free survival is “practice-changing” and may represent a “paradigm shift” in treatment, according to experts.
Biktarvy was found both effective and safe for long-term use in HIV, as well as for patients with mental health conditions.
Integrating immunotherapies will continue to improve patient outcomes and quality of life.

Christina Madison, PharmD, FCCP, AAHIVP, sat down with Amy Marie Merrell, to discuss current issues within sexual health.
Study data suggest that both vaccines induce similar functional antibody responses against pneumococcal serotype 3.
Among individuals who received systemic antibiotics within 8 weeks, 6 months, 12 months, and 24 months of receiving fecal microbiota, RBX2660 prevented recurrence.
This finding underlines the need for methods that address multiple genomic alterations and targeted therapies effective in tumors driven by variations in suppressor genes or transcription factors.
The biosimilar landscape is growing, but the FDA should leverage analytical data to efficiently determine the quality of these products and propel them toward approval, according to a session at the Academy of Managed Care Pharmacy’s Nexus 2023 conference.

Approximately 96% physicians feel it is important to assess patients aged 60 years and older for cognitive impairment, but 48% of these patients have no had assessments from their physician.

The FDA is the only authority that can designate a biosimilar as interchangeable with its reference product, which requires that the product has similar clinical results for the same indication.

There are 3 main medication-related issues for mental health care, which includes polypharmacy, nonadherence to prescribed medication, and underutilization of medications.

Areas of focus for specialty medications include biosimilars, cancer treatments, and drugs across disease states such as inflammatory conditions, HIV, and multiple sclerosis.
Twice-daily tenapanor tablets are recommended for patients with chronic kidney disease who are on dialysis, unresponsive or intolerant of any dose of a phosphate binder therapy.
Following the results of a meta-analysis that reviewed biosimilar trial design and endpoints, investigators urge for greater biosimilar uptake initiatives.

The approval marks the first and only once-daily target therapy for generalized myasthenia gravis for self-administration in this patient population.

Grace Yun, PharmD, and pharmacy manager at St. Francis Hospital, discussed the value of the pharmacist and how she sees the future of pharmacy evolving.

A specialist in adult, child, and adolescent psychiatry discusses the challenges in ADHD treatment for both patients and providers, as well as insights from a recent survey of health care professionals.

In the clinical setting, psychedelic medicine has promise as a safe and efficacious treatment for PTSD, TBIs, and other mental health issues.

PLCOm2012 uses additional parameters to predict the risk of lung cancer and was found more effective when identifying both Indigenous and non-Indigenous individuals with lung cancer.

As pharmacists provide more health care services, opportunities arise for more sophisticated patient engagement and billing solutions that drive patient satisfaction and increased loyalty.

Recent FDA findings highlight that a major ingredient in popular cold fighters, phenylephrine, may not offer genuine relief.
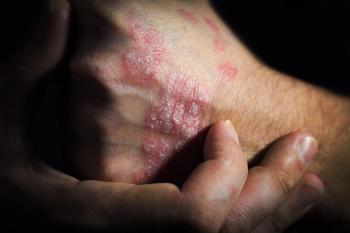
Bimekizumab is a novel, humanized monoclonal IgG1 antibody that potently and selectively neutralizes both IL-7A and IL-17F, which are key cytokines driving inflammatory processes in plaque psoriasis.

Artificial intelligence and machine learning can significantly benefit managed care pharmacy, specifically in contract reading and interpretation.