
Apomorphine (Kynmobi) is indicated for the acute, intermittent treatment of off episodes in patients with Parkinson disease.

Apomorphine (Kynmobi) is indicated for the acute, intermittent treatment of off episodes in patients with Parkinson disease.
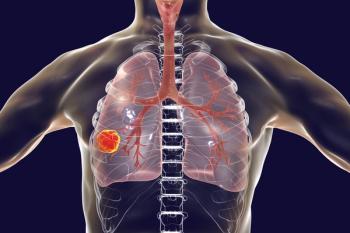
About one-third of participants in the phase 1b cohort of the KRYSTAL-1 study show an intracranial response, according to Mirati Therapeutics.

The nature of CLL being a long-term, incurable disease makes its treatment a challenge as it is more focused toward improving patient’s quality of life.

With the rapid pace of change in biosimilar payer benefit design, efforts to stay regularly updated on policy changes ahead of time become critical for patient care.

Experts discuss the pros and cons of using oral azacytidine to treat patients with acute myeloid leukemia.

With a lack of head-to-head studies comparing combination regimens in frontline metastatic renal cell carcinoma, deciding on how to choose the right regimen can pose a challenge.

The FDA approved tirzepatide for the treatment of type 2 diabetes on May 13, 2022

Crizotinib has also been approved to treat non-small cell lung cancer that has spread to other parts of the body and is caused by a defect in either the ALK or ROS1 gene.

Ryan Burke, PharmD, director of professional affairs at Pharmacy Technician Certification Board, discusses more advanced roles for pharmacy technicians and how they can continue to grow beyond their current roles.

Investigators argue that much of the language around women who give birth later in life is rooted in ableism and ageism and is out of step with current childbirth trends.

Provepharm filed its application with the agency in September 2021, and, with the new approval, its US affiliate will market the product directly in the United States.

People who regularly fasted for decades had lower risk of hospitalization or death from COVID-19, adding to the potential health benefits of intermittent fasting.

Pursuing the expanded access process for treatment may be the best option for patients who have exhausted all available FDA-approved drugs and clinical trials.

Triptans are contraindicated in patients with ischemic heart disease, cerebrovascular disease, uncontrolled hypertension, and peripheral artery disease.
The objective of this study is to evaluate the use of low-dose pioglitazone, without relying on high doses of insulin, by enhancing sensitivity as a suitable, cost-effective strategy compared to larger insulin doses in patients with limited access to care.
Patient education, naloxone training, and monitoring programs make a difference.

In 2020, there was a 17% decrease in new HIV diagnoses and significant reductions in HIV testing in health care and non-health care settings, impeding the Ending the HIV Epidemic initiative to increase testing and reduce infections.

A recent case report detailing multiple procedures and inconclusive biopsies underwent by a patient with unusual presentation of immunoglobulin G4-related disease before conclusive diagnosis emphasizes the importance of follow-up with patients with suspicion of systemic disease.

Dutch biopharmaceutical company Byondis submitted the BLA for [vic-]trastuzumab duocarmazine (SYD985) in the treatment of patients with HER2-positive metastatic breast cancer with the goal of improving patient outcomes.
A pharmacist’s role in the ambulatory care setting has traditionally included a review of medications for potential drug-drug interactions and adverse events, medication reconciliation, and the provision of counseling services.

NVX-CoV2373, a protein-based COVID-19 vaccine, may increase the country's vaccination rate for those hesitant to receive other vaccines.

Each technology should be evaluated on its own merit and, importantly, in appropriate context for your organization given other resources.

With a coordinated, interprofessional team supporting them, patients are empowered with information at the point-of-care to help manage medication therapy problems as they arise.

Moderna is completing regulatory submissions for mRNA-1273.214 in preparation for the fall booster season, based on clinical data showing that the booster candidate has a significantly higher neutralizing antibody response against all tested variants including Omicron BA.4/5.

The FDA has approved fam-trastuzumab deruxtecan-nxki (Enhertu) for previously treated HER2-positive breast cancer and gastric or gastroesophageal junction adenocarcinoma.

The FDA's current way of inspecting facilities for quality control is badly outdated and is politically unworkable, unreliable, and unsafe.

A booster dose of both Omicron-adapted vaccine candidates elicited a substantially higher immune response against Omicron BA.1.

Methotrexate is an immunosuppressive agent used to treat cancer, dermatology-, and rheumatology-related issues.

Vibhuti Arya Amirfar, PharmD, MPH, discusses building individual and systems resilience to dismantle structural racism in the pharmacy field.