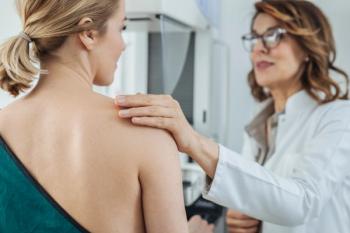
The screenings should be conducted every other year, beginning at age 40 and continuing through age 74, according to the new recommendation.

Kennedy Ferruggia is an associate editor at Pharmacy Times®. She graduated from The College of New Jersey in 2023 in journalism and marketing. Prior to this position, she worked as a pharmacy associate for community pharmacies.

The screenings should be conducted every other year, beginning at age 40 and continuing through age 74, according to the new recommendation.

Vitamin D deficiency (VDD) is an important factor in the pathogens of schizophrenia.

Trastuzumab is indicated for adjuvant breast cancer, metastatic breast cancer, and gastric cancer.
The severity of RSV disease among adults further highlights how crucial vaccine polices and recommendations are.
A 76% lower risk was displayed with adjuvant alectinib compared to chemotherapy treatment for non–small cell lung cancer.

Retinoic acid, an active form of vitamin A, is crucial for the stem cells to depart lineage plasticity which get separated into hair cells or epidermal cells in vitro.

The primary endpoint of the study was clinical remission at week 52 with vedolizumab
Individuals treated with chemotherapy following a breast cancer diagnosis were more at risk of developing second primary lung cancer.
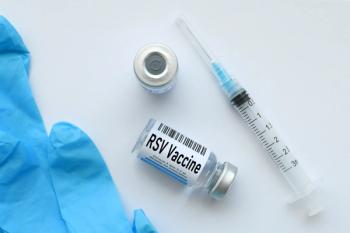
The results displayed well tolerability and safety with Abrysvo, meeting the study’s co-primary endpoint of immunogenicity and primary safety
Treatment with LYT-200 is currently being assessed in a phase 1/2 adaptive design trial in advanced/metastatic solid tumors and in a phase 1b clinical trial.
The designation was granted based on overall survival data from an ongoing randomized phase 2 clinical trial.
Both dual primary endpoints—progression-free survival and overall survival—were met with durvalumab.

Aged mice displayed improvements in their spatial cognition, short-term memory, and muscle durability.

Study outcomes demonstrated that 80 HER2-postive BTC individuals enrolled in Cohort 1 of the study achieved a confirmed objective response rate of 41.3%
The positive results in both progression-free survival and overall response rate were endorsed in a confirmatory study.

Stephanie Ashraf, MD, offered clinical experience in managing insomnia at the American Medical Women’s Association (AMWA) 2024 Annual Meeting.

The recent ODD for the utidelone injectable could offer another treatment option for patients with BCBM.
The results displayed a dramatic reduction in the individuals’ tumors within days after single treatment.
Calder’s 3D Vaxlock technology attained 11 times more effective immune response, compared to typical comparator.
The adjuvanted RSVPreF3 vaccine could prevent nearly 3 million symptomatic RSV-ARI cases among US older adults over 3 years’ time, according to study results.

Benefits throughout post-progression endpoints and outcomes were reported for osimertinib in combination with chemotherapy.
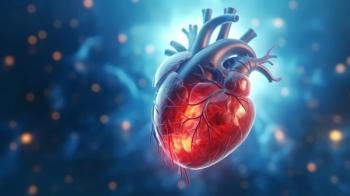
All 20 participants in a 12-month follow-up study attained acute success from ablation procedures.
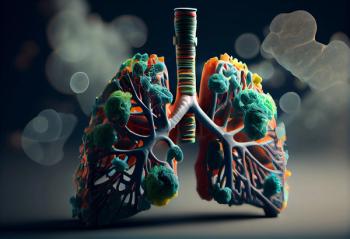
Pembrolizumab in combination with chemotherapy followed by maintenance olaparib did not meet its pre-specified statistical criteria of overall survival or progression free survival.
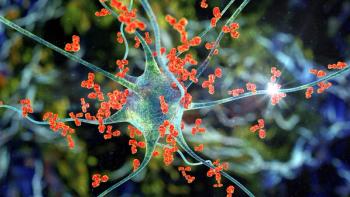
Zero relapses were reported among individuals that received ravulizumab-cwvz over the 73 weeks of treatment.
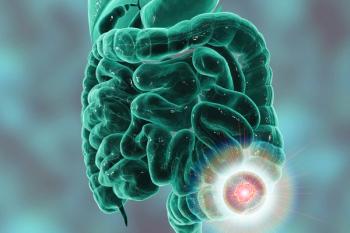
The test’s sensitivity in distinguishing colorectal cancer, in combination with real-world adherence, showed its ability to detect cancer at a curable stage.

The platform is a first-of-its-kind therapeutic approach to promote remyelination among individuals with relapsing/remitting multiple sclerosis.
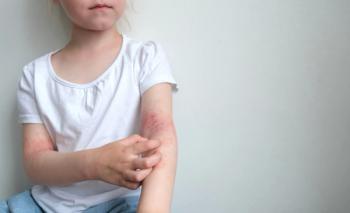
The findings suggest that ruxolitinib cream could aid itch, pain, and sleep disturbance.

Individuals included in the study reported positive changes in fatigue, and disease activity displayed endoscopic and histologic remission.

In the State of the Union Address, President Biden said he wants to expand the $2,000 out-of-pocket cap beyond seniors and allow Medicare to negotiate more costly drugs.
They also compared the approach of the European Medicines Agency and the FDA in drug approvals.