Study results show that individuals who were vaccinated against the flu were 37% less likely to be hospitalized and 82% less likely to be admitted to the ICU that those who were unvaccinated.
Ashley Gallagher is an editor at Pharmacy Times®. She graduated from St. Bonaventure University in 2020 in journalism and mass communications. Previously, she worked as a pharmacy technician for a retail chain.

Study results show that individuals who were vaccinated against the flu were 37% less likely to be hospitalized and 82% less likely to be admitted to the ICU that those who were unvaccinated.

Northwestern Medicine study results show that mice were protected from other strains when those from prior infections or in their vaccinations were greater than 70%.

Stacy Miller, senior content management consultant for Wolters Kluwer Health, discusses how mental health in the United States could be improved by stronger connections between prescribers and pharmacists.

Aysha Akhtar, CEO and co-founder for the Center for Contemporary Sciences discusses the FDA Modernization Act of 2021, aiming to modernize the drug testing process by allowing the use of other testing methods as an alternative to animal testing.
The agency has a 2-day meeting scheduled for this week for an independent advisory panel to discuss the available data.
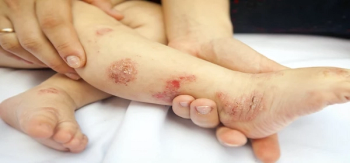
Mark Becker, the CEO of Vivacare, discusses how health care professionals can counsel patients on eczema treatment and care so they can help to deter patients from googling their symptoms and developing what can be termed “medical anxiety.”
Early intervention with ocrelizumab resulted in a 35% reduction of risk in individuals with RMS who needed a walking aid over 7 and a half years.
New study results show that previously infected patients had molecular markers that suggested that immune cells could last longer and migrate more effectively to the respiratory tract.

Washington State University study results show that slumber-deprived individuals had more difficulty reducing the emotions they felt when so instructed than those who slept normally.
A phase 3 trial met the primary endpoint, and the long-acting antibody combination reduced symptoms or death by 67% for patients compared with those who took a placebo.
AbbVie announced positive top-line results from a phase 3 trial for the efficacy and safety of upadacitinib for individuals with the rheumatic disease.
The PROpel phase 3 trial met the primary endpoints of radiographic progressing-free for men with metastatic castration-resistant prostate when treated with Olaparib, combined with abiraterone.
The medication, which has been cleared as a treatment for the inflammatory disease of the throat, is under priority review by the agency.
The medication has both short- and long-term effects on improving excessive sleepiness.
Anna Legreid-Dopp, PharmD, Senior Director of Clinical Guidelines and Quality Improvement at the American Society of Health-System Pharmacists discusses Pfizer’s request to approve their COVID-19 vaccine for children 5 to 11.

Avacopan (Tavneos, ChemoCentryx) met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks during a phase 3 trial in patients with severe active anti-neutrophil cytoplasmic autoantibody-associated vasculitis.
Investigators from the University of Houston find that 45% of the positive samples were from the soles of shoes.
CAR-NK therapies are also shown to have fewer adverse reactions, and 25% of mice that were treated remained disease-free.
Those who are younger than aged 40 years tend to regain these senses at higher rates than those who are older.
A town hall meeting with representatives from the agencies emphasized the effectiveness and likelihood of getting a third dose of the vaccine.

New legislation in New York state seeks to address flaws in prescription drug distribution system.

In a study, individuals who were taking prucalopride had better memory tests and fMRI scans indicating enhanced brain activity in relation to cognition.
AZD7442 (AstraZeneca), a long-acting antibody combination, reduced the risk of developing symptomatic COVID-19 by 77% compared to placebo.

Labcorp’s collection kit can simultaneously detect the coronavirus and influenza A/B in individuals aged 2 years and older, and results can be determined in 1 to 2 days.
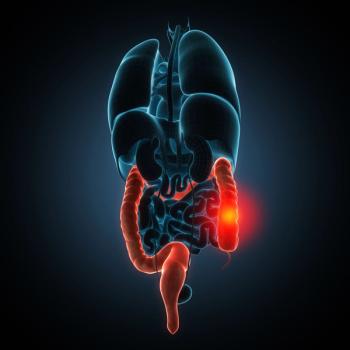
Study results show KNT-127 is a promising therapeutic option and enhances the anti-inflammatory response.

With the Delta variant, there was an increase in coronavirus-related hospitalizations by more than one-third among these individuals.

Axsome Therapeutics continues to explore its narcolepsy drug after the FDA granted it orphan drug designation.

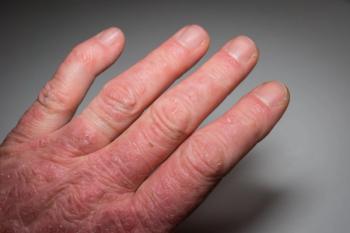
In a Pharmacy Times® interview, Jessica Hui, a researcher and pediatric allergist at National Jewish Health, discussed how increased hand hygiene may be harming the hands of healthcare workers.
The main factors behind the vaccine hesitancy are the potential adverse effects post-vaccination and effect on their autoimmune conditions, as well as lack of trial data, an analysis shows.

The medication receives the approval after it was shown to be more effective than daily tenofovir disoproxil fumarate with emtricitabine tablets.