Merck will also conduct clinical trials in both males and females to evaluate the efficacy of a single dose of their current human papillomavirus vaccine compared with the approved 3-dose regimen.
Ashley Gallagher is an editor at Pharmacy Times®. She graduated from St. Bonaventure University in 2020 in journalism and mass communications. Previously, she worked as a pharmacy technician for a retail chain.

Merck will also conduct clinical trials in both males and females to evaluate the efficacy of a single dose of their current human papillomavirus vaccine compared with the approved 3-dose regimen.

Celebrities, local advocates, patients, and pharmacists can effectively promote public health initiatives and raise disease awareness by authentically leveraging trusted relationships.

Previously, the study met the other primary endpoint of progression free survival, showing statistically significant and clinically meaningful improvements compared to chemoradiotherapy alone.

The efficacy data are consistent with other phase 3 studies, reinforcing lebrikizumab's potential as a first-line treatment across a range of skin tones.

The agency will focus on collaboration to protect public health; advancing regulatory approaches; developing standards, guidelines, and best practices; and supporting research that evaluates and monitors AI performance.

Oteseconazole (Vivjoa; Mycovia Pharmaceutical Inc) is the first and only medication for this indication in postmenopausal women and those who do not have reproductive potential.

Senators Ron Wyden and Mike Crapo reaffirm their support on 2 health care bills that passed the United States Senate Committee on Finance in 2023.
High bacterial load in cerebrospinal fluid was associated with unfavorable outcomes and death for adults with pneumococcal meningitis.

Investigators found that 1,1,1 trichloroethane; 2-nitropropane; and 2,4,6 trichlorophenol were significantly associated with asthma symptoms for 3 exposure periods.
The pneumococcal vaccine was most likely associated with a higher risk of immunological events for those who were immunocompromised

The study showed no clinical differences for remission or asthma at age 28 years based on lung function, body mass index, daily smoking, exposure to parental tobacco smoke, or house dampness .
Furthermore, the study authors indicated that patients with lymphoma and community-acquired pneumonia have broader dysregulated responses.

This podcast episode discussed the impact of celebrity endorsements on trends in weight loss drugs like Ozempic, body image issues, and the role of pharmacists in educating patients on appropriate use of GLP-1 medications for weight management and chronic diseases.

Semaglutide (Wegovy; Novo Nordisk) reduces the risk of cardiovascular death, heart attack, and stroke in adults with cardiovascular disease and either obesity or overweight when combined with the standard-of-care.
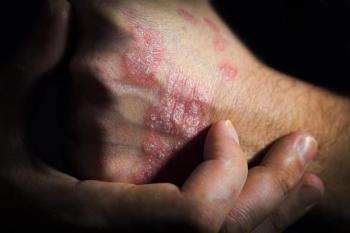
The biosimilarity was evident between SB17 (Samsung Bioepis), ustekinumab (Stelara; Janssen Immunology), and those switching from ustekinumab to SB17.

Cardinal Health released its 2024 biosimilars report, including research and in-depth analyses on legislative development, payer landscapes, and new treatments in the pipeline.

Individuals with persistent asthma and at least 1 complex comorbidity were more likely to use telemedicine, the results of the study showed.

The study is a companion study to the ROCKstar study, which investigated the drug in individuals with cGVHD who received 2 to 5 prior lines of therapy.

Tocilizumab-aazg (Tyenne; Fresenius Kabi) is the first approved biosimilar to tocilizumab (Actemra; Genentech) as both intravenous and subcutaneous.
A phase 1 study previously demonstrated that MW032 and denosumab were bioequivalent in pharmacokinetics, pharmacodynamics, safety, and immunogenicity.

Artificial intelligence (AI) recommendations resulted in fewer recurrent strokes, heart attacks, or vascular death within 3 months of a previous ischemic stroke.

This episode highlights how celebrity endorsements and local influencers can be leveraged to address vaccine hesitancy by shaping attitudes, but also must account for factors like audience trust and the potential for both benefits and limitations.

The continuous glucose monitoring system will be available for purchase online and without a prescription starting in summer 2024.

Denosumab-bbdz (Wyost; Sandoz) and denosumab-bbdz (Jubbonti; Sandoz) are approved as interchangeable for all indications of denosumab (Xgeva and Prolia; Amgen).
Investigators also found that those with community-acquired, radiology-confirmed pneumonia with lower respiratory tract infections had worse outcomes.
In the study, investigators found superior kidney outcomes with injectable semaglutide (Ozempic) for individuals with type 2 diabetes and chronic kidney disease.

Laws can help eliminate common barriers to transparency but are not the only way to achieve health care transparency.

Juvéderm Voluma XC (AbbVie) is the first and only hyaluronic acid dermal filler to receive FDA approval for this indication with results lasting up to 13 months.

AAO-HNSF guideline development group included 17 panel members, including experts from otolaryngology and allergy, those who have knowledge in clinical practice guideline development, and patient representatives.

Investigators at Duke University and the University of North Carolina determine what causes recurrent UTIs, even after antibiotics cleared the bacterial infection.