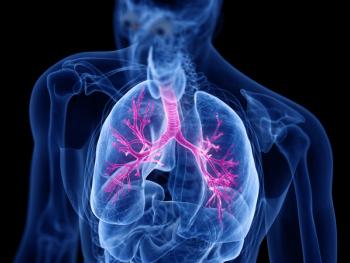
Dupilumab (Dupixent; Sanofi) showed greater reductions in mucus plugs and greater improvements in airway volume and flow for individuals with uncontrolled moderate-to-severe asthma.
Ashley Gallagher is an editor at Pharmacy Times®. She graduated from St. Bonaventure University in 2020 in journalism and mass communications. Previously, she worked as a pharmacy technician for a retail chain.

Dupilumab (Dupixent; Sanofi) showed greater reductions in mucus plugs and greater improvements in airway volume and flow for individuals with uncontrolled moderate-to-severe asthma.
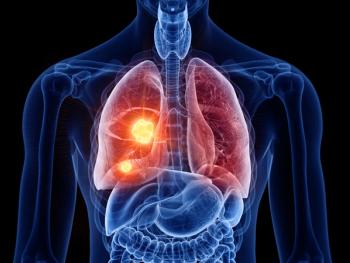
The FDA also granted traditional approval to amivantamab-vmjw for adults whose disease has progressed on or after platinum-based chemotherapy.

Walgreens will start dispensing the abortion pill as soon as a week.
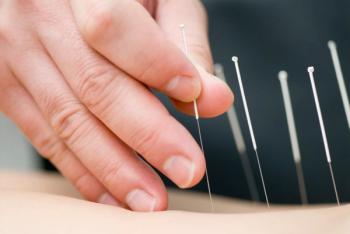
The effects of acupuncture were independent of sex, age, medication use, and coexisting conditions, according to the results of the study published in BMJ Open.

The investigators included 24 studies across 7 countries, including Australia, Canada, New Zealand, Norway, Singapore, Sweden, and the United States.

Investigators determined that the vaccination reduced the prevalence of vaccine serotypes with an effectiveness of 89.6% based on the study results.

VK2735, a dual agonist of glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide, is being developed as a potential treatment for various metabolic disorders.
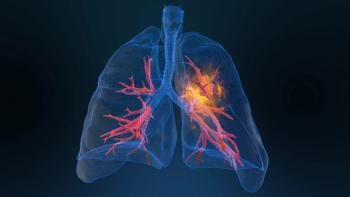
NVL-520 is a novel brain-penetrant ROS1-selective TKI being investigated for ROS1-positive metastatic non–small cell lung cancer in patients who have previously been treated with 2 or more ROS1 TKIs.
If approved, epcoritamab (DuoBody CD3xCD20; AbbVie, Genmab) would be the first and only subcutaneous bispecific antibody for treatment in this patient population.

In LePage v. Center for Reproductive Medicine, the Alabama Supreme Court ruled that “extrauterine embryos” are considered children, causing some infertility clinics to halt IVF processes.

The podcast highlights addressing misinformation, the impact of celebrity endorsements in pharmaceuticals, and the important role of pharmacists in educating patients.

The data follow the FDA approval of omalizumab for the reduction of allergic reactions, including anaphylaxis, that can occur after exposure to 1 or more foods.
The results were presented as a late-breaking poster presentation at the American Academy of Allergy, Asthma, and Immunology Annual Meeting.
The new indication includes individuals with HIV who have suppressed viral loads with known or suspected M184V/I resistance.
The sNDA was based on results from the KRYSTAL-1 study, evaluating the drug alone or in combination with other anti-cancer therapies in those with advanced solid tumors who have KRASG12C mutations.
Adalimumab-ryvk is the first high-concentration, citrate-free biosimilar to Humira that has been granted interchangeability status for the 40 mg/0.4 ml injection.
As of January 2024, Keytruda has been approved for 6 indications in NSCLC across the metastatic and earlier stages of the disease.
Osimertinib was previously approved as a monotherapy, the first-in-line global standard of care, for non–small cell lung cancer indications.
This marks the first and only time that an individualized T cell therapy has been approved by the FDA for a solid tumor cancer.
Tigilanol tiglate is a novel, small molecule drug for the intratumoral treatment of solid tumors, inducing tumor cell death and stimulating immune response.
This frequency can allow for faster observation of toxicities and adherence issues.
Cardiac Solutions, a physician-owned practice specializing in personalized treatment plans, used RevUp Remote Patient Monitoring and Chronic Care Management to achieve these cardiovascular readmission rates.

If approved, this would mark the first MDMA-assisted therapy and psychedelic-assisted therapy approved, calling for a reschedule of MDMA from Schedule I. A PDUFA was set for this summer.
Bepirovirsen is a triple action investigational antisense oligonucleotide and is being evaluated in the B-Well phase 3 clinical trial program for the treatment of chronic hepatitis B.

The FDA approval marks the first and only FDA-approved oral therapy for this patient population, and the drug is expected to be available by the end of February.

The levels of disease activity after 6 months of tumor necrosis factor-α inhibitors could help predict the response to therapy at 4 years for those who have long-standing rheumatoid arthritis.

In addition to managing adverse effects and educating both patients and providers, pharmacists help patients get access to much-needed treatments.

Bevacizumab is a recombinant humanized G1 monoclonal anti-vascular endothelial growth factor antibody that is used in combination with chemotherapy to treat solid tumors.
Investigators found high incidences of non-susceptibility with penicillin and increasing resistance to macrolides for pneumococcal diseases.
Ibrutinib and other Bruton tyrosine kinase inhibitors can cause the development of or worsen pre-existing hypertension.