
Investigators compared the reference products of adalimumab (Humira; AbbVie) and etanercept (Enbrel; Amgen) with biosimilar products.
Ashley Gallagher is an editor at Pharmacy Times®. She graduated from St. Bonaventure University in 2020 in journalism and mass communications. Previously, she worked as a pharmacy technician for a retail chain.

Investigators compared the reference products of adalimumab (Humira; AbbVie) and etanercept (Enbrel; Amgen) with biosimilar products.
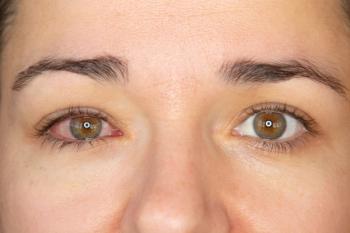
Caution Patients Against the Use of Redness-Reducing Eye Drops.

Pharmacy Times will be at the American Diabetes Association (ADA) 84th Scientific Sessions in Orlando, Florida from June 21 to June 24, 2024.

Impaired glucose tolerance is a significant risk factor for developing diabetes.
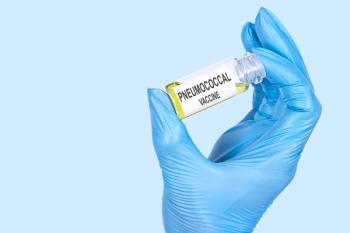
V116 (Capvaxive; Merck) elicited higher immune responses than the comparator for the serotypes that are unique to the vaccine, according to studies submitted to the FDA.
Mim8 had a safe and well-tolerated profile, with no deaths or thrombotic events reported for patients with hemophilia A.
Brentuximab vedotin in combination with lenalidomide and rituximab had a favorable safety profile for patients with relapsed/refractory diffuse large B-cell lymphoma.
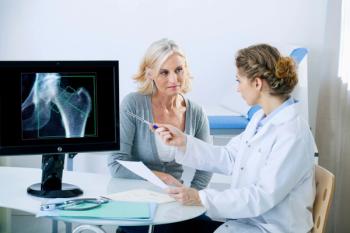
The new findings were presented as an oral presentation at the 2024 European Calcified Tissue Society Congress, held from May 25, 2024, to May 28, 2024.

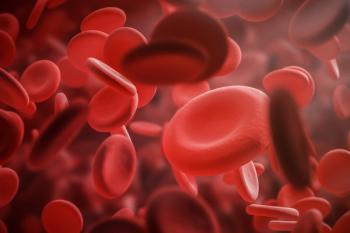
Individuals with very low- to intermediate-risk myelodysplastic syndromes treated with luspatercept (Reblozyl; Bristol Myers Squibb) achieved improvements in hemoglobulin levels.
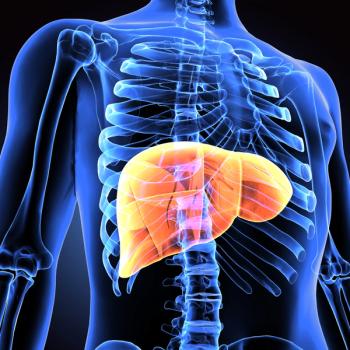
Elafibranor (Iqirvo; GENFIT) is a first-in-class, once daily oral peroxisome proliferator-activated receptor for the treatment of primary biliary cholangitis.
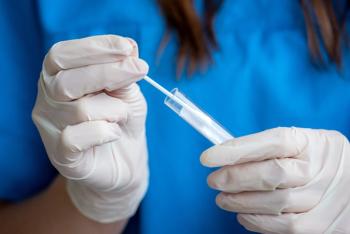
The 4-in-1 respiratory diagnostic test is either a single nasopharyngeal or anterior nasal swab that can confirm or rule out SARS-CoV-2, influenza A virus, influenza B virus, and respiratory syncytial virus with a single test.
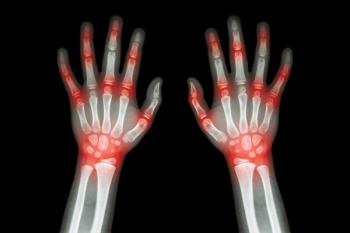
Polyarticular juvenile idiopathic arthritis is a form of arthritis that affects multiple joints at one time.

To qualify for therapy, patients must be screened for type 1 diabetes islet autoantibodies when they are asymptomatic, which requires better education on screening opportunities.
Serotypes associated with V116 (Merck & Co. Inc.) had higher clinical and economic burden for individuals with invasive pneumococcal disease.
By improving health insurance coverage and cost of inhalers, such as out-of-pocket caps, access to care can be increased. Some companies are aiming to improve access by implementing price caps.

Mirikizumab met both co-primary end points and all secondary end points at week 52, according to results presented at Digestive Disease Week in Washington DC.
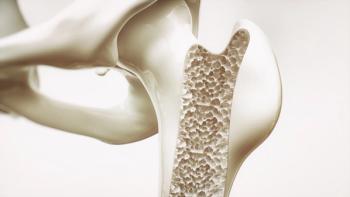
FKS518 (Fresenius Kabi) would be indicated for the treatment of osteoporosis in men and women, including glucocorticoid-induced osteoporosis and bone loss due to prostate or breast cancer.
For patients with a blood eosinophil count of 150 cells/µL or greater, tezepelumab had a significant reduction of approximately 37% in the rate of moderate or severe exacerbations.
Lisocabtagene maraleucel is being investigated for mantle cell lymphoma, relapsed or refractory (R/R) large B-cell lymphoma, and R/R follicular lymphoma.

Approximately 40% of caregivers make dosing errors when administering liquid medication to children.

Body mass index is the main driver of type 2 diabetes globally, accounting for 52.2% of disability-adjusted life years.

In prior studies, higher levels of physical activity were associated with higher prevalence of coronary artery calcium.

Providers, the community, health systems, and health policies can all play a part in addressing various challenges in care for obesity.

This is Moderna's second mRNA vaccine approval and the first mRNA vaccine approved for an indication other than COVID-19.

Pediatric patients with rheumatic disease, such as systemic lupus erythematosus, mixed connective tissue disease, juvenile dermatomyositis, and systemic vasculitis are at an increased risk of serious infection.

Topline results of QWINT-2 and QWINT-4 met the primary end point of change in hemoglobin A1c compared to degludec and glargine, respectively, for those with type 2 diabetes.
Lisocabtagene maraleucel (Breyanzi; Bristol Myers Squibb) is a CD19-directed CAR T-cell therapy that is delivered as a one-time infusion.
Eculizumab-aeeb (Bkemv; Amgen) is approved for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.

Added data from the SELECT trial helps to increase confidence in safety and efficacy of semaglutide, a glucagon-like peptide 1 medication.