Publication
Article
Pharmacy Times
Counsel Patients on New Antiobesity Medications
Older treatments and novel options are changing the way obesity is treated and managed
Since this article was written, the FDA has approved tirzepatide in adults with obesity or overweight plus 1 weight-related comorbidity.
In 2013, the American Medical Association designated obesity as a chronic disease.1
Image credit: Jarun011 | stock.adobe.com

Rates of obesity have been increasing over the past decade; in the United States, the percentage of adults aged 20 and older with obesity is 41.9%.2 However, recent advances in antiobesity medications (AOMs) can change the way we treat this chronic condition.
SCREENING
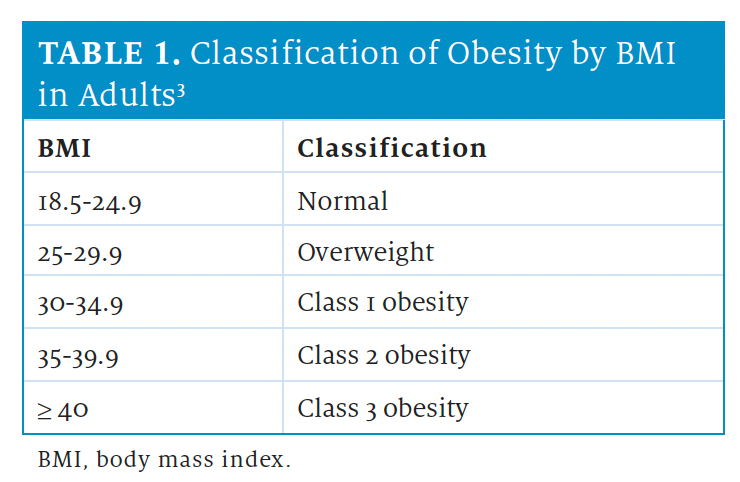
AOMs are indicated in individuals with a body mass index (BMI) of 30 or higher, or with a BMI of 27 or higher in the presence of 1 or more comorbidities.3
Waist circumference should also be considered in patients with a BMI of 25 to 35. Waist circumferences of at least 102 cm (approximately 40 in) in men and at least 88 cm (approximately 35 in) in women indicate abdominal obesity, which is associated with increased risk for adiposityrelated disease.3 See the Table3 for classification of obesity by BMI in adult populations.
NEWER AOMS
FDA-approved AOMs include the GLP-1 receptor agonists semaglutide (Ozempic; Novo Nordisk) or liraglutide (Victoza; Novo Nordisk); combination phentermine/topiramate extended release (Trokendi XR; Supernus Pharmaceuticals, Inc); combination bupropion/naltrexone (Contrave; Currax Pharmaceuticals LLC); orlistat (Xenical; Cheplapharm Arzneimittel GmbH); and sympathomimetic stimulant medications such as phentermine (Adipex-P; Teva Pharmaceuticals USA), benzphetamine (Didrex; Pfizer Inc), phendimetrazine (Bontril; Mallinckrodt Inc), and diethylpropion (Tenuate; Nostrum Laboratories, Inc). Some medications are also being used off-label for weight loss.
Semaglutide is injected subcutaneously once weekly, with dose titration every 4 weeks to the target dose. The doses increase from 0.25 mg once weekly during the first month to 0.5 mg once weekly during month 2, then 1 mg once weekly during month 3, and 1.7 mg once weekly beginning month 4. The maximum dose could optionally increase to 2.4 mg. In clinical trials, semaglutide was associated with significant weight loss by 68 weeks, which was maintained at 2 years.4
Liraglutide is injected subcutaneously once daily, with dose titration once weekly to target dose. Patients increase the dose by 0.6 mg each week until 3 mg is reached. Liraglutide was associated with significant and sustained weight loss at 20 weeks and 56 weeks.5
Semaglutide was associated with greater weight loss (mean weight loss, 16% at 68 weeks) than liraglutide (mean weight loss, 6% at 68 weeks). Common adverse effects (AEs) of both drugs were nausea, diarrhea, vomiting, constipation, and abdominal pain. Contraindications include pregnancy, history of pancreatitis, or a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia 2.4,5
OFF-LABEL AOMS
Tirzepatide (Mounjaro; Eli Lilly and Company) is a once-weekly subcutaneous dual-acting GLP-1 and GIP receptor agonist approved for type 2 diabetes mellitus (DM), but it is not FDA approved for obesity. Patients start with 2.5 mg once weekly for 4 weeks and, at week 5, increase to 5 mg once weekly for 4 weeks. At week 9, the dose can be raised to 7.5 mg per week. Tirzepatide is associated with more significant weight loss than semaglutide in patients with obesity and type 2 DM.6 Common AEs include dose-related abdominal pain, nausea, diarrhea, and constipation.6
OLDER AOMS
Combination weight loss medications include phentermine/topiramate extended release and bupropion/naltrexone. Phentermine/topiramate extended release induced weight loss in the first year but was less effective in the second year. AEs of phentermine/topiramate extended release include dry mouth, constipation, paresthesia, cognitive dysfunction, and increased risk of kidney stones. Phentermine/topiramate extended release is contraindicated in pregnancy.7
Combination bupropion/naltrexone caused modest weight loss (approximately 5%) but was associated with increased blood pressure and heart rate. Contraindications include pregnancy, chronic opioid use, uncontrolled hypertension, seizure disorder, and eating disorders.7
Orlistat alters fat absorption and is administered orally 3 times daily. Mean weight loss with orlistat across multiple clinical trials was approximately 8%. Orlistat frequently causes cramps, flatus, fecal incontinence, and oily spotting, which limits its tolerability.8
Sympathomimetic stimulants include phentermine, benzphetamine, phendimetrazine, and diethylpropion, which are approved for shortterm use of up to 12 weeks. Sympathomimetics stimulants have significant AEs, including increased blood pressure and heart rate, dry mouth, insomnia, and potential for abuse.
CONCLUSION
In the next few years, we can expect more novel AOMs to come to market, with the potential to change the way we treat obesity. The choice of AOMs must take into consideration the patient’s comorbidities, drug contraindications, and expected degree of weight loss and improvements in cardiorenal and metabolic risk.
ABOUT THE AUTHOR
Jean Covino, DHSC, MPA, PA-C is clinical professor and department chairperson in the Pace University College of Health Professions.
Jennifer Hofmann, DMSC, MS, PA-C is director of didactic education at Pace University's NYC Physician Assistant Program.
REFERENCES
- Recognition of obesity as a disease. American Medical Association House of Delegates. NPR. Accessed October 15, 2023. https://media.npr.org/documents/2013/jun/ama-resolutionobesity.pdf
- National Health and Nutrition Examination Survey. CDC. Accessed October 15, 2023. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?Cycle=2017-2020
- Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63(25 pt B):2985-3023. doi:10.1016/j.jacc.2013.11.004
- Wegovy. Prescribing information. Novo Nordisk Inc; 2023. Accessed October 15, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215256s007lbl.pdf
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138-150. doi:10.1001/jama.2021.23619
- Frías JP, Davies MJ, Rosenstock J, et al; SURPASS-2 Investigators. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515. doi:10.1056/NEJMoa2107519
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605.doi:10.1016/S0140-6736(10)60888-4
- Leblanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care–relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(7):434-447.doi:10.7326/0003-4819-155-7-20111040-00006

Newsletter
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.






